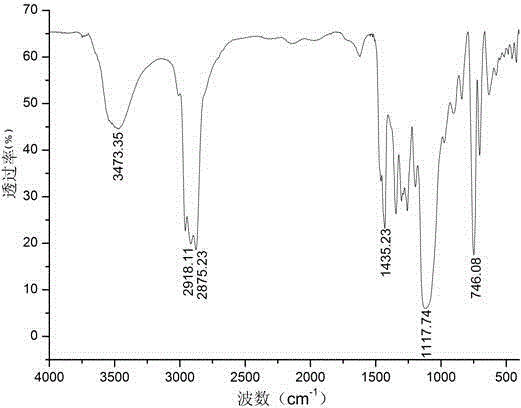
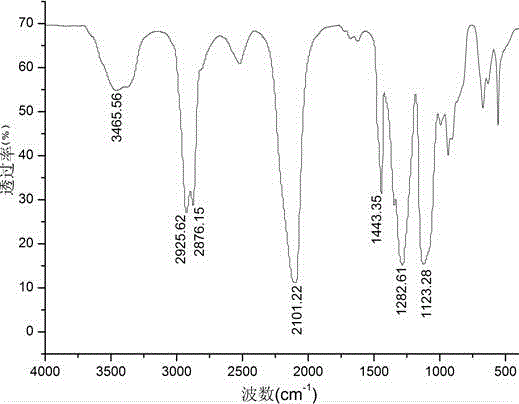
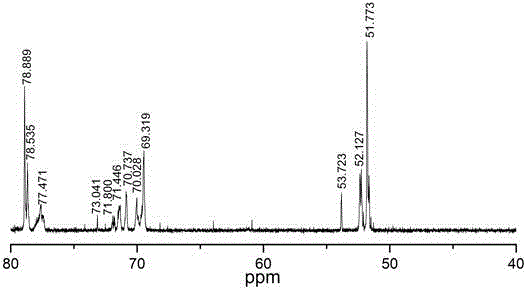
Six-arm type hydroxyl-terminated glycidyl azide polymer preparation method
A technology of glycidyl ether and terminal hydroxyl group, which is applied in the field of organic chemical synthesis, can solve the problem of few types of multi-arm type ester-terminated polyazido glycidyl ethers, etc., and achieves good thermal stability and improves the effect of thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add sorbitol and catalyst to the solvent dichloromethane, stir, keep a constant temperature of 30°C, add epichlorohydrin dropwise, and react for 3 hours after the dropwise addition to obtain a polyepichlorohydrin solution; the mixture of sorbitol and epichlorohydrin The molar ratio is 1:90; the mass fraction of the catalyst is 2.5% of the total mass of sorbitol and epichlorohydrin.
[0023] Wash the polyepichlorohydrin solution with deionized water, discard the upper aqueous solution, and distill the organic matter in the lower layer under reduced pressure to obtain six-arm polyepichlorohydrin; under nitrogen protection, at a temperature of 120 ° C, the six-arm polyepoxychlorohydrin Propane and sodium azide were synthesized in N,N-dimethylformamide solvent for 12 hours, and the molar ratio of polyepichlorohydrin to sodium azide was 1:1.1; polyepichlorohydrin was based on theoretical epichlorohydrin Mole count. After the reaction, most of the N,N-dimethylformamide solve...
Embodiment 2
[0033]Add sorbitol and the catalyst into the solvent dichloromethane, stir, and keep a constant temperature of 30°C; add epichlorohydrin dropwise, and react for 3 hours after the dropwise addition to obtain polyepichlorohydrin solution B. The molar ratio of sorbitol and epichlorohydrin is 1:60, and the mass fraction of the catalyst is 2.5% of the total mass of sorbitol and epichlorohydrin.
[0034] Wash the polyepichlorohydrin solution with deionized water, discard the upper aqueous solution, and distill the organic matter in the lower layer under reduced pressure to obtain six-arm polyepichlorohydrin; Propane and sodium azide were synthesized in N,N-dimethylformamide solvent for 16 hours, and the molar ratio of polyepichlorohydrin to sodium azide was 1:1.1; polyepichlorohydrin was based on theoretical epichlorohydrin In terms of moles, after the reaction, most of the N,N-dimethylformamide solvent was evaporated under reduced pressure at 50°C, then washed with deionized water,...
Embodiment 3
[0038] Add sorbitol and catalyst to the solvent dichloromethane, stir, keep a constant temperature of 30°C, add epichlorohydrin dropwise, and react for 3 hours after the dropwise addition to obtain a polyepichlorohydrin solution; the mixture of sorbitol and epichlorohydrin The molar ratio is 1:48, and the mass fraction of the catalyst is 2.5% of the total mass of sorbitol and epichlorohydrin.
[0039] Wash the polyepichlorohydrin solution with deionized water, discard the upper aqueous solution, and distill the organic matter in the lower layer under reduced pressure to obtain six-arm polyepichlorohydrin; Propane and sodium azide were synthesized in N,N-dimethylformamide solvent for 12 hours, and the molar ratio of polyepichlorohydrin to sodium azide was 1:1.0; polyepichlorohydrin was based on theoretical epichlorohydrin In terms of moles, after the reaction, most of the N,N-dimethylformamide solvent was evaporated under reduced pressure at 50°C, then washed with deionized wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Onset decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com