Method for separating cobalt, nickel and magnesium from nickel oxide ore pickle liquor
A technology of nickel oxide ore and separation method, applied in the field of separation of magnesium, cobalt and nickel in acid leaching solution of nickel oxide ore, can solve the problems of increasing the cost of extraction and separation, increasing the cost of extraction, prolonging the extraction process, etc. Separation efficiency, achieve rapid precipitation, and speed up filtration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
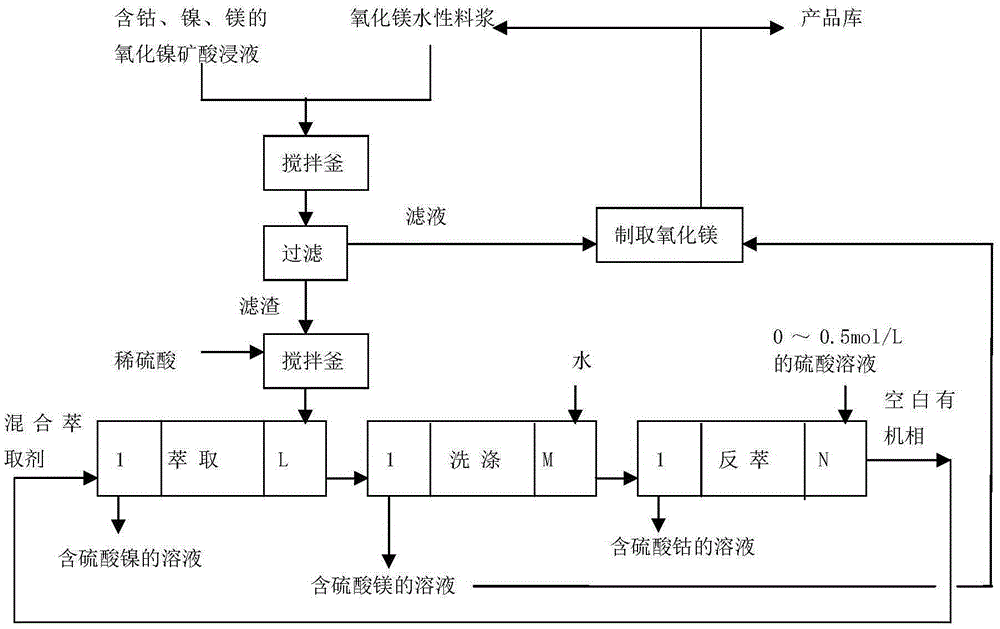
[0038] (1) Co in the nickel oxide ore sulfuric acid leaching solution 2+ ,Ni 2+ , Mg 2+ The concentrations are 0.1g / L, 1.05g / L, 2.3g / L, iron ions (including Fe 2+ And Fe 3+ , The same below) the concentration is 0.001g / L, the pH value of the material liquid is 1.2, and the material liquid is sent to the stirred tank;
[0039] Weigh 0.001 times the mass of the nickel oxide mineral acid leaching solution, and add water to prepare an aqueous slurry of magnesium oxide with a mass concentration of 40% magnesium oxide;
[0040] Start the agitator of the stirred tank (rotational speed is 250 rpm), then add the aqueous magnesium oxide slurry to the stirred tank at a flow rate of 0.3 L / min, stir and react at 30°C for 3 hours to obtain a mixture (pH value of 8.1);
[0041] (2) Liquid-solid separation is performed on the mixture obtained in step (1) to obtain solid filter residue and filtrate;
[0042] (3) Transfer the filter residue obtained in step (2) to another stirring tank, add 0.5 mol / L su...
Embodiment 2
[0048] (1) Co in the nickel oxide ore sulfuric acid leaching solution 2+ ,Ni 2+ , Mg 2+ Concentrations are 0.3g / L, 2.3g / L, 13g / L, the concentration of iron ions is 0.005g / L, the pH of the material liquid is 2.2, and the material liquid is sent to the stirred tank;
[0049] Weigh 0.0018 times the mass of the nickel oxide mineral acid leaching solution and add water to prepare an aqueous slurry of magnesium oxide with a mass concentration of 40% magnesium oxide;
[0050] Start the agitator of the stirred tank (speed of 350rpm), then add the aqueous magnesium oxide slurry to the stirred tank at a flow rate of 0.8L / min, stir and react at 50°C for 2.5h to obtain a mixture (pH value of 8.2);
[0051] (2) Liquid-solid separation is performed on the mixture obtained in step (1) to obtain solid filter residue and filtrate;
[0052] (3) Transfer the filter residue obtained in step (2) to another stirred tank, add 0.8 mol / L sulfuric acid solution, and heat to 30°C and stir until the filter residu...
Embodiment 3
[0057] (1) Co in the nickel oxide ore sulfuric acid leaching solution 2+ ,Ni 2+ , Mg 2+ The concentrations of the are 0.3g / L, 5.7g / L, 30g / L, the concentration of iron ions is 0.0004g / L, the pH of the material liquid is 3.1, and the material liquid is sent to the stirred tank;
[0058] Weigh 0.003 times the mass of the nickel oxide mineral acid leaching solution, and add water to prepare an aqueous slurry of magnesium oxide with a mass concentration of 50% magnesium oxide;
[0059] Start the agitator of the stirred tank (speed of 400rpm), then add the aqueous magnesium oxide slurry to the stirred tank at a flow rate of 0.5L / min, stir and react at 70°C for 1h to obtain a mixture (pH value of 8.3);
[0060] (2) Liquid-solid separation is performed on the mixture obtained in step (1) to obtain solid filter residue and filtrate;
[0061] (3) Transfer the filter residue obtained in step (2) to another stirred tank, add 1mol / L sulfuric acid solution, and heat to 60°C and stir until the filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com