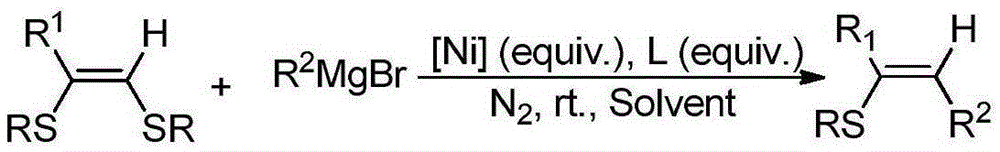Synthetic method for sulfo trisubstituted alkenes compound
A synthetic method and tri-substitution technology, applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of expensive use, increased operation difficulty and production cost, etc., and achieve high selectivity and yield, wide application range of substrates, and raw materials. A wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0017] Add 0.2 mol (Z)-1,2-diphenylthiostyrene, 1.0 mol methylmagnesium bromide and 0.2 mol NiCl to a 10 mL reaction tube 2 and 0.2molPPh 3 and 2 mL of dry THF, under the protection of nitrogen, the reaction was carried out at room temperature for 10 h. After the reaction was completed, a small amount of methanol was slowly added to quench the reaction, an appropriate amount of silica gel powder was added, the solvent was removed, and 1-phenylthio-2-methylstyrene was separated by column chromatography with a yield of 46%.
preparation example 2
[0019] Add 0.2mol (Z)-1,2-diphenylthiostyrene, 1.0mol methylmagnesium bromide and 0.2molPPh to a 10mL reaction tube 3 and 2 mL of dry THF, under the protection of nitrogen, the reaction was carried out at room temperature for 10 h. After the reaction was completed, a small amount of methanol was slowly added to quench the reaction, an appropriate amount of silica gel powder was added, and the solvent was removed, but no expected product was formed.
preparation example 3
[0021] Add 0.2 mol (Z)-1,2-diphenylthiostyrene, 1.0 mol methylmagnesium bromide and 0.2 mol NiCl to a 10 mL reaction tube 2 and 2 mL of dry THF, under the protection of nitrogen, the reaction was carried out at room temperature for 10 h. After the reaction was completed, a small amount of methanol was slowly added to quench the reaction, an appropriate amount of silica gel powder was added, and the solvent was removed, but no expected product was formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com