Modified benzophenone photo-initiator and preparation method thereof
A technology of benzophenone and photoinitiator, which is applied in the field of benzophenone photoinitiator and its preparation, can solve the problems of easy migration, decreased solubility, and restrictions on the application of photocuring systems, so as to accelerate the photopolymerization reaction , avoid the decrease of solubility, and curb the effect of oxygen inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
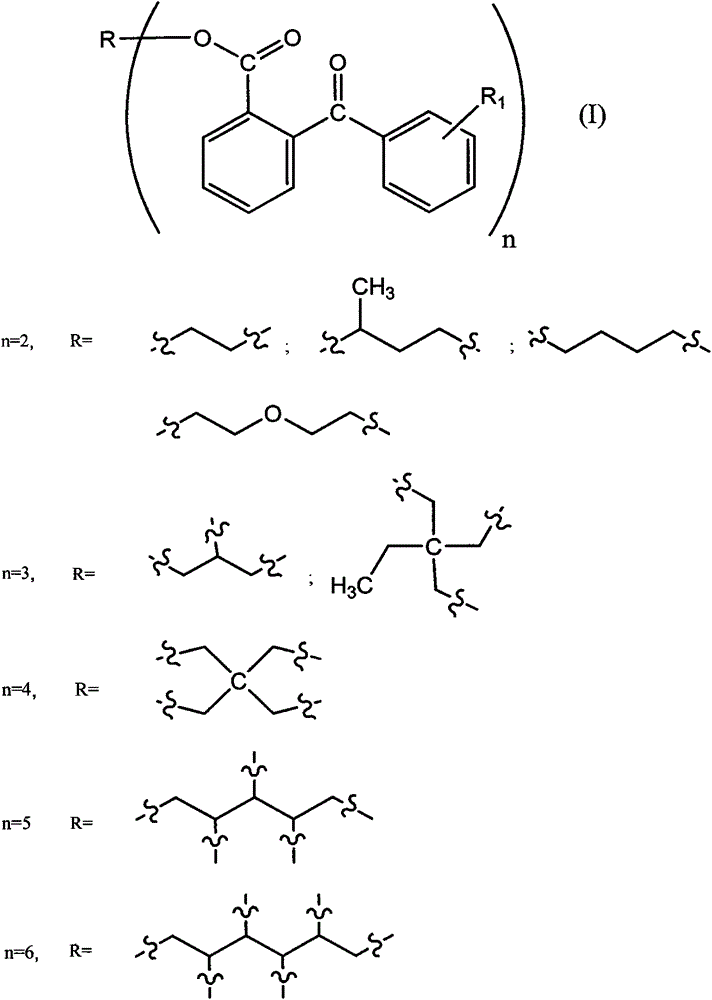
[0031] The preparation method of the modified benzophenone photoinitiator of a kind of formula (I) proposed by the present invention, comprises the steps:
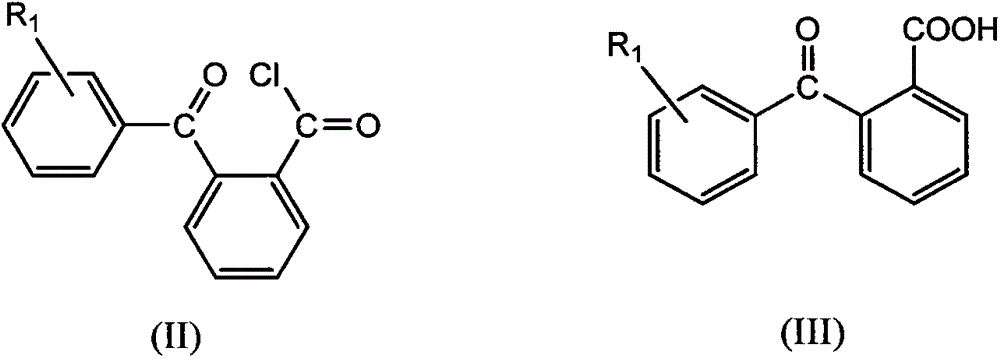
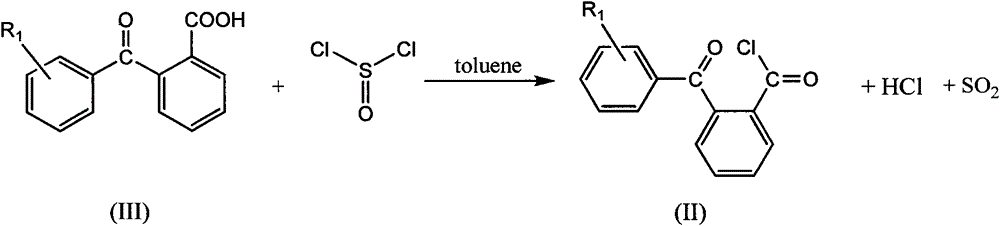
[0032] (a) get 2-(benzoyl) benzoic acid (339.2g, 1.5mol) and join in the 1000ml three-necked flask filled with 300ml of toluene, stir and dissolve at 60 DEG C, slowly add dichlorosulfuric acid dropwise to this mixed system Sulfone (111.2ml, 1.5mol), refluxed for 6 hours after the dropwise addition, the solvent toluene was removed by rotary evaporation to a colorless liquid (342.6g), which was 2-(benzoyl)benzoyl chloride, the intermediate Compound II, the yield was 93.3%.
[0033] 1 HNMR (300MHz, CDCl 3 ): δ8.32 (d, 1H, -CHCCOCl), δ7.81 (d, 2H, -CHCCO), δ8.03 (d, 1H, -CHCCO), δ7.61-7.73 (m, 3H).
[0034] Elemental analysis: (C 14 H 9 ClO 2 )
[0035]
Embodiment 1
[0037] (b) Pentaerythritol (34.1 g, 0.25 mol) was added to a 500 ml three-necked flask containing 200 ml of 1,2-dichloroethane, and 100 ml of triethylamine was added simultaneously. Take the product 2-(benzoyl)benzoyl chloride (244.8g, 1mol) in step (a) and slowly add it dropwise to the above mixed system, stir and react at 40°C for 12 hours, filter, and remove by rotary evaporation Solvent 1,2-dichloroethane to obtain 231.7 g of colorless liquid, which is one of the products of the present invention: I-1 or G-BP-4, with a yield of 83.8%.
[0038] 1 HNMR (300MHz, CDCl 3 ): δ4.21(s, 8H, -CH 2 ), δ8.28(d, 4H, -CHCCOO), δ7.81(d, 8H, -CHCCO), δ8.01(d, 4H, o-phCOO), δ8.45(t, 4H, p-phCOO ).
[0039] Elemental analysis: (C 61 H 44 O 12 )
[0040]
Embodiment 2
[0042](b) Methylolpropane (33.5g, 0.25mol) was added to a 500ml three-necked flask containing 200ml of 1,2-dichloroethane, and 100ml of triethylamine was added simultaneously. Take the product 2-(benzoyl)benzoyl chloride (183.6g, 0.75mol) in step (a) and slowly add it dropwise to the above mixed system, stir and react at 40°C for 12 hours, filter, and rotate by evaporation The solvent 1,2-dichloroethane was removed to obtain 170.1 g of a colorless liquid, which was one of the products of the present invention: I-2 or G-BP-3, with a yield of 88.6%.
[0043] 1 HNMR (300MHz, CDCl 3 ): δ0.93 (t, 3H, -CH 3 ), δ1.73 (q, 2H, -CH 2 CH 3 ), δ4.59(s, 6H, -CH 2 ), δ7.83 (d, 6H, -CHCCO), δ8.18 (d, 3H, o-phCOO), δ8.41 (t, 3H, p-phCOO).
[0044] Elemental Analysis (C 48 H 38 O 9 ):
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com