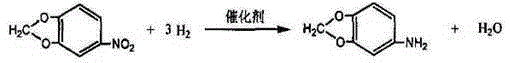3,4-methylenedioxy aniline preparation method
A technology of methylenedioxybenzene and dioxyaniline, which is applied in the field of chemical synthesis, can solve the problems of low yield, environmental pollution of metal reduction, and less application, and achieves high product yield, less three-waste treatment, and selectivity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 25% dilute nitric acid with a pure amount of 81.9g (1.3mol) into the reaction tank, and heat up to a certain temperature. The selection of the temperature rise to a certain temperature is shown in Table 1; slowly add 63.65 g (0.52mol) 1,2-methylenedioxybenzene, while vigorously stirring at a speed of 400r / min, keep warm for 0.5 hours after the dropwise addition, cool down to 20°C and filter to obtain the wet product 3,4-methylenedioxy The nitrobenzene was washed with purified water until the pH value was neutral, and dried at 40°C under a vacuum of 0.09 MPa to obtain the 3,4-methylenedioxynitrobenzene intermediate. The yield and purity are shown in Table 1. Put 3,4-methylenedioxynitrobenzene, skeletal nickel catalyst and ethanol into the autoclave in turn, then discharge the air, start to feed hydrogen when the temperature rises to 60°C, and continue the hydrogen reaction under 2.5MPa pressure for 2h, Then pass hydrogen intermittently until the hydrogen pressure rem...
Embodiment 2
[0020] Add a pure amount of 81.9g (1.3mol) of 25% dilute nitric acid into the reaction tank, raise the temperature to 50-60°C, and slowly add 63.65g (0.52mol) of 1,2-methylenedioxy to the reaction tank at a certain speed Benzene, the selection of the speed of adding 1,2-methylenedioxybenzene is shown in Table 2; at the same time, it is vigorously stirred at a speed of 400r / min. The product 3,4-methylenedioxynitrobenzene was washed with purified water until the pH value was neutral, and dried at 40°C under a vacuum of 0.09MPa to obtain the intermediate of 3,4-methylenedioxynitrobenzene. The yield and purity are shown in Table 2. Put 3,4-methylenedioxynitrobenzene, skeletal nickel catalyst and ethanol into the autoclave in turn, then discharge the air, start to feed hydrogen when the temperature rises to 60°C, and continue the hydrogen reaction under 2.5MPa pressure for 2h, Then pass hydrogen intermittently until the hydrogen pressure remains constant, filter the catalyst, and ...
Embodiment 3
[0024] Add a pure amount of 81.9g (1.3mol) of 25% dilute nitric acid into the reaction tank, raise the temperature to 50-60°C, and slowly add 63.65g (0.52mol) of 1,2-methylene to the reaction tank at a speed of 0.4g / min Dioxybenzene, stirring vigorously at a certain speed at the same time, the selection of the stirring speed is shown in Table 3; after the dropwise addition, keep warm for 0.5 hours, cool to 20°C and filter to obtain the wet product 3,4-methylenedioxynitrate benzene, washed with purified water until the pH value is neutral, and dried at 40°C under a vacuum of 0.09 MPa to obtain 3,4-methylenedioxynitrobenzene intermediate. The yield and purity are shown in Table 3. Put 3,4-methylenedioxynitrobenzene, skeletal nickel catalyst and ethanol into the autoclave in turn, then discharge the air, start to feed hydrogen when the temperature rises to 60°C, and continue the hydrogen reaction under 2.5MPa pressure for 2h, Then pass hydrogen intermittently until the hydrogen p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com