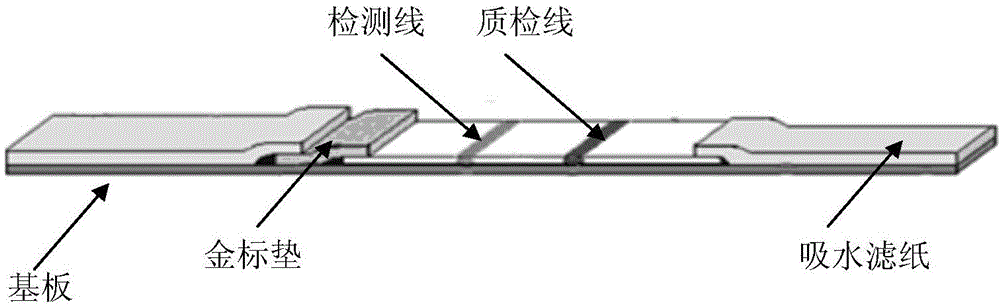
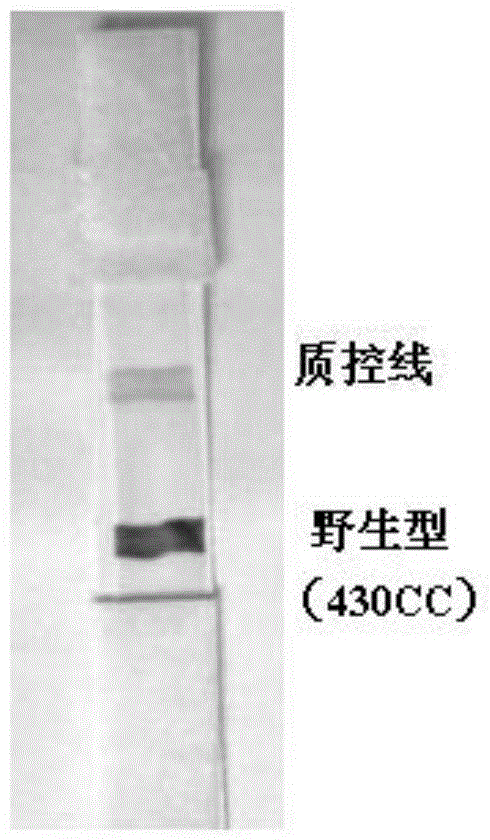
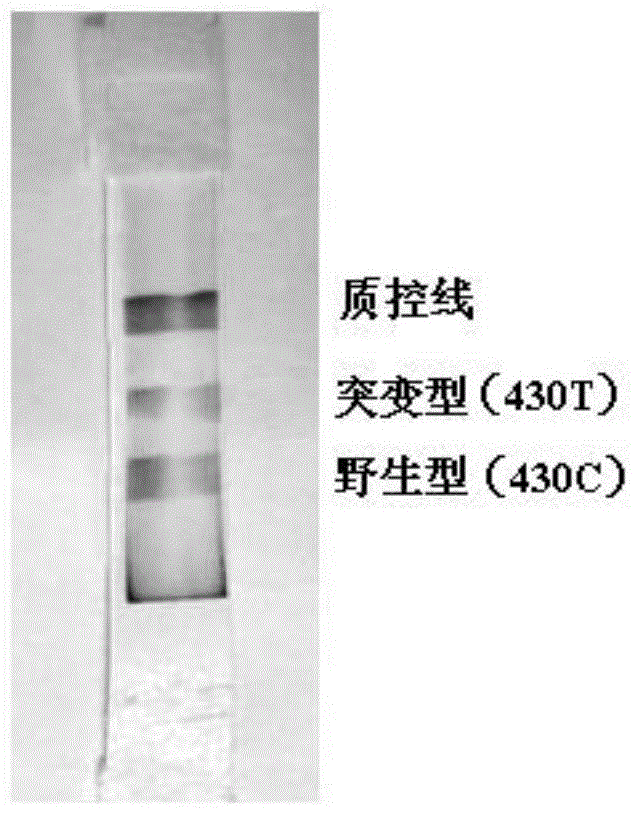
Nucleic acid detection method and test strip
A detection method and nucleic acid technology, which is applied in the field of nucleic acid detection methods and test strips, and can solve problems such as complex operation, high false positives, and easy contamination of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: the preparation of test strip of the present invention
[0080] 1. Preparation of streptavidin solution coated with colloidal gold
[0081] Prepare a streptavidin solution with a concentration of 1ug / uL. Inject the streptavidin solution into the dialysis card and place it in a beaker filled with ddH2O for dialysis. Take out the dialysis card, take out the dialysis sample from the card, measure the protein concentration, and confirm the concentration is 1ug / uL.
[0082] The pH of the colloidal gold solution not coated with streptavidin was detected, and the pH was adjusted to 6.6. The optimal reaction volume of colloidal gold solution and streptavidin solution is 100uL: 1ug (20uL). Streptavidin was added dropwise into the colloidal gold solution, oscillating while adding, and left to stand at room temperature for 30 minutes after the addition was complete. 2000rpm, 4°C, 10min. The supernatant was collected, 10000rpm, 10min, 4°C, and 10mM Tris was used ...
Embodiment 2
[0088] Embodiment 2: the preparation of test strip of the present invention
[0089] 1. Preparation of streptavidin solution coated with colloidal gold
[0090] Prepare a streptavidin solution with a concentration of 1ug / uL. Inject the streptavidin solution into the dialysis card and place it in the ddH 2 O beaker for dialysis. Take out the dialysis card, take out the dialysis sample from the card, measure the protein concentration, and confirm the concentration is 1ug / uL.
[0091] The pH of the colloidal gold solution not coated with streptavidin was detected, and the pH was adjusted to 6.6. The optimal reaction volume of colloidal gold solution and streptavidin solution is 100uL: 1ug (20uL). Streptavidin was added dropwise into the colloidal gold solution, oscillating while adding, and left to stand at room temperature for 30 minutes after the addition was complete. 2000rpm, 4°C, 10min. The supernatant was collected, 10000rpm, 10min, 4°C, and 10mM Tris was used to diss...
Embodiment 3
[0097] Embodiment 3: the preparation of test strip of the present invention
[0098] 1. Preparation of streptavidin solution coated with colloidal gold
[0099] Prepare a streptavidin solution with a concentration of 1ug / uL. Inject the streptavidin solution into the dialysis card and place it in the ddH 2 O beaker for dialysis. Take out the dialysis card, take out the dialysis sample from the card, measure the protein concentration, and confirm the concentration is 1ug / uL.
[0100] The pH of the colloidal gold solution not coated with streptavidin was detected, and the pH was adjusted to 6.6. The optimal reaction volume of colloidal gold solution and streptavidin solution is 100uL: 1ug (20uL). Streptavidin was added dropwise into the colloidal gold solution, oscillating while adding, and left to stand at room temperature for 30 minutes after the addition was complete. 2000rpm, 4°C, 10min. The supernatant was collected, 10000rpm, 10min, 4°C, and 10mM Tris was used to diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com