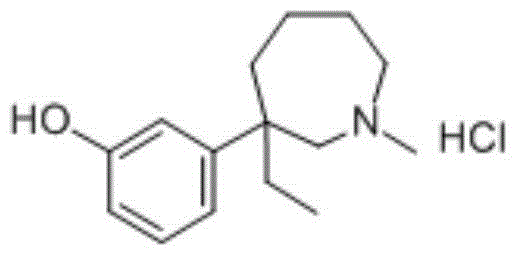
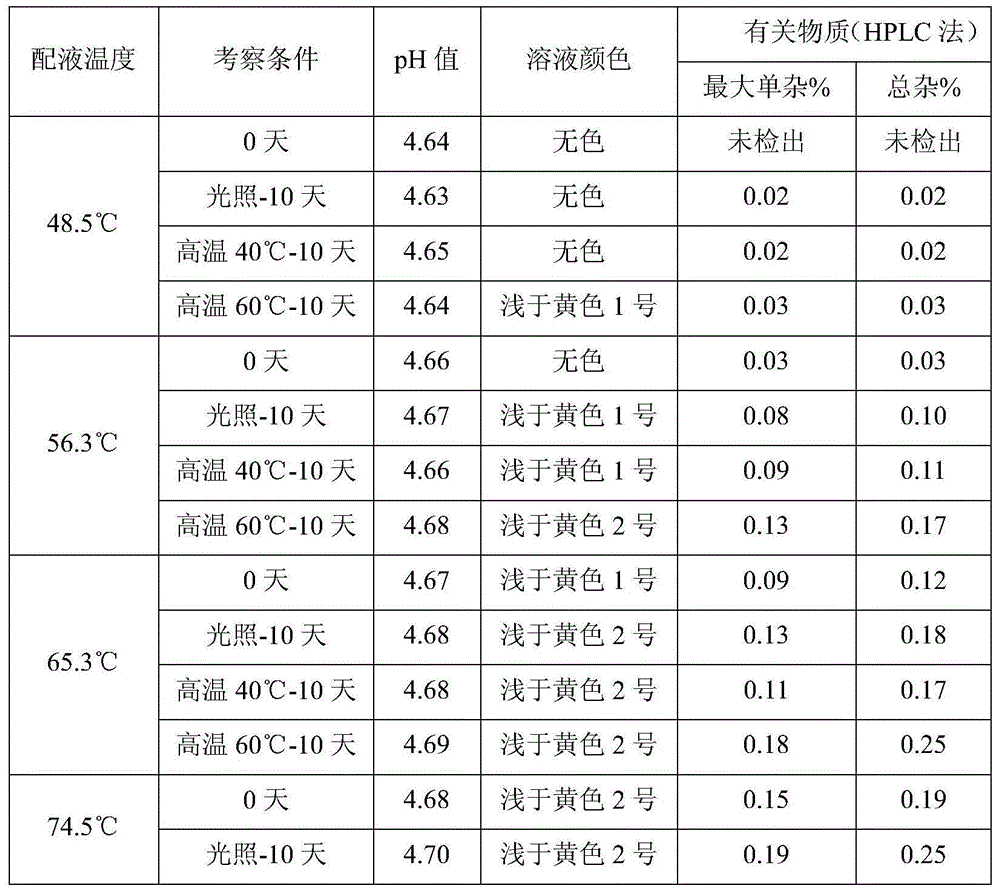
Meptazinol hydrochloride injection medicine composition and preparation method thereof
A technology of meptafol hydrochloride and meptafol, which is applied in the field of medicine, can solve problems such as patient irritation, hemolysis risk, and safety hazards, and achieve the effects of avoiding degradation, improving product quality, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 Mebutamol Hydrochloride Injection Prescription (in 1000 pieces)
[0046] prescription
Dosage
Mebutamol Hydrochloride (calculated as Mebutaol)
100g
glucose
50g
1ml
Sodium acetate
1g
Water for Injection
Add to 1000ml
[0047] Preparation Process:
[0048]Take 800ml of water for injection, control the temperature of the water for injection at 40°C, fill with nitrogen until the dissolved oxygen content of the water for injection is lower than 2.0mg / L, add 100g of meptafol hydrochloride (calculated as meptafol), 50g of glucose, 1ml acetic acid and 1g sodium acetate, stirred to dissolve completely, added water for injection to 1000ml; then added 0.3g activated carbon, stirred and adsorbed for 20min; "Membrane" series-connected sterilizing filter for fine filtration; control the filling nitrogen flow rate to 15L / min, and fill each 1.0ml bottle into a 1ml low borosilicate glas...
Embodiment 2
[0049] Example 2 Mebutamol Hydrochloride Injection Prescription (in 1000 pieces)
[0050] prescription
Dosage
Mebutamol Hydrochloride (calculated as Mebutaol)
100g
glucose
50g
1ml
Sodium acetate
5g
Water for Injection
Add to 1000ml
[0051] Preparation Process:
[0052] Take 800ml of water for injection, control the temperature of the water for injection at 40°C, fill with nitrogen until the dissolved oxygen content of the water for injection is lower than 2.0mg / L, add 100g of meptafol hydrochloride (calculated as meptafol), 50g of glucose, 1ml acetic acid and 5g sodium acetate, stirred to dissolve completely, added water for injection to 1000ml; then added 0.3g activated carbon, stirred and adsorbed for 20min; "Membrane" in series with sterilizing filter for fine filtration; control the nitrogen flow rate of potting to 14L / min, and pot each 1.0ml into a 1ml low borosilicate glass ampoule...
Embodiment 3
[0053] Example 3 Mebutamol Hydrochloride Injection Prescription (in 1000 pieces)
[0054] prescription
Dosage
Mebutamol Hydrochloride (calculated as Mebutaol)
100g
glucose
50g
2ml
Sodium acetate
3g
Water for Injection
Add to 1000ml
[0055] Preparation Process:
[0056] Take 800ml of water for injection, control the temperature of the water for injection at 45°C, fill with nitrogen until the dissolved oxygen content of the water for injection is lower than 2.0mg / L, add 100g of meptafol hydrochloride (calculated as meptafol), 50g of glucose, 2ml acetic acid and 3g sodium acetate, stirred to dissolve completely, added water for injection to 1000ml; then added 0.3g activated carbon, stirred and adsorbed for 20min; "Membrane" in series with the sterilization filter for fine filtration; control the flow rate of nitrogen gas for potting to 12L / min, and fill each 1.0ml bottle into a 1ml low boro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com