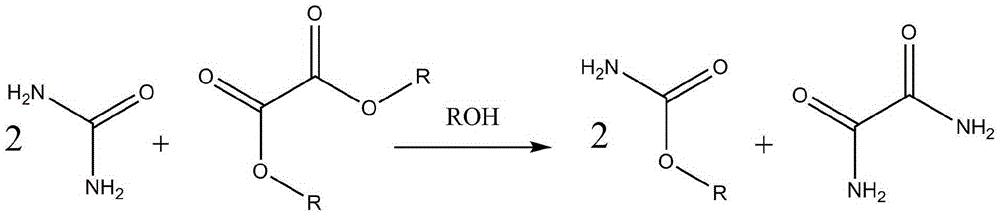
Process method of co-production of oxamide and carbamic acid ester through ammonia ester exchange method
The technology of carbamate and process method is applied in the field of co-production of oxamide and carbamate by transesterification method, which can solve the problems of by-products affecting the reaction process and single product, and avoid the increase of process and equipment costs. , the effect of uninhibited conversion, avoiding process and equipment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
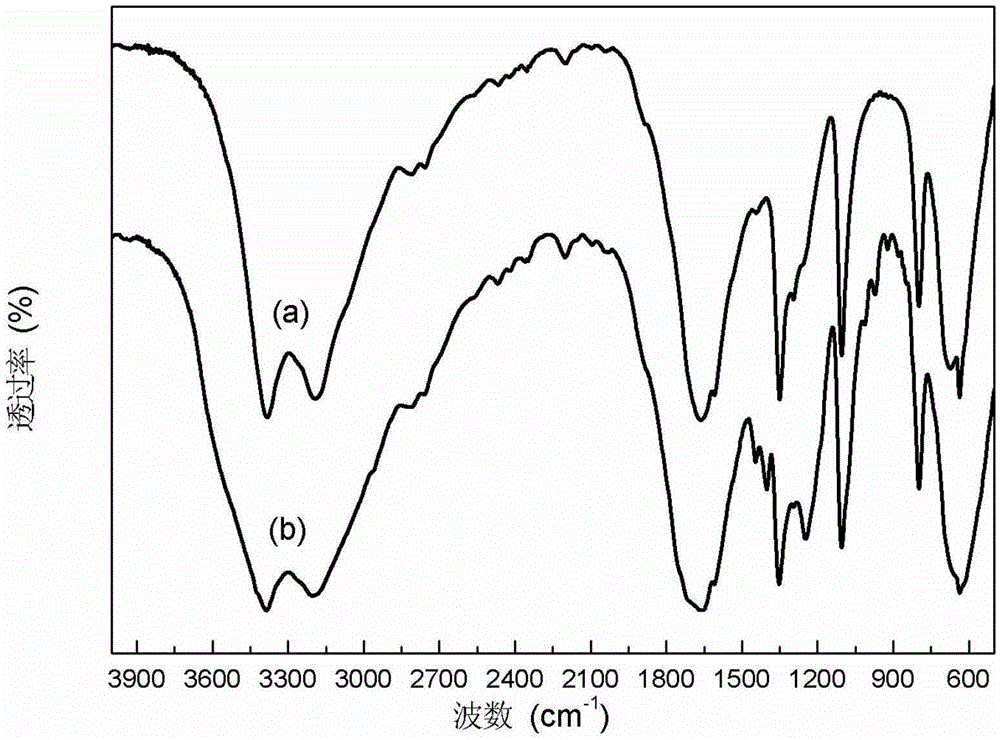
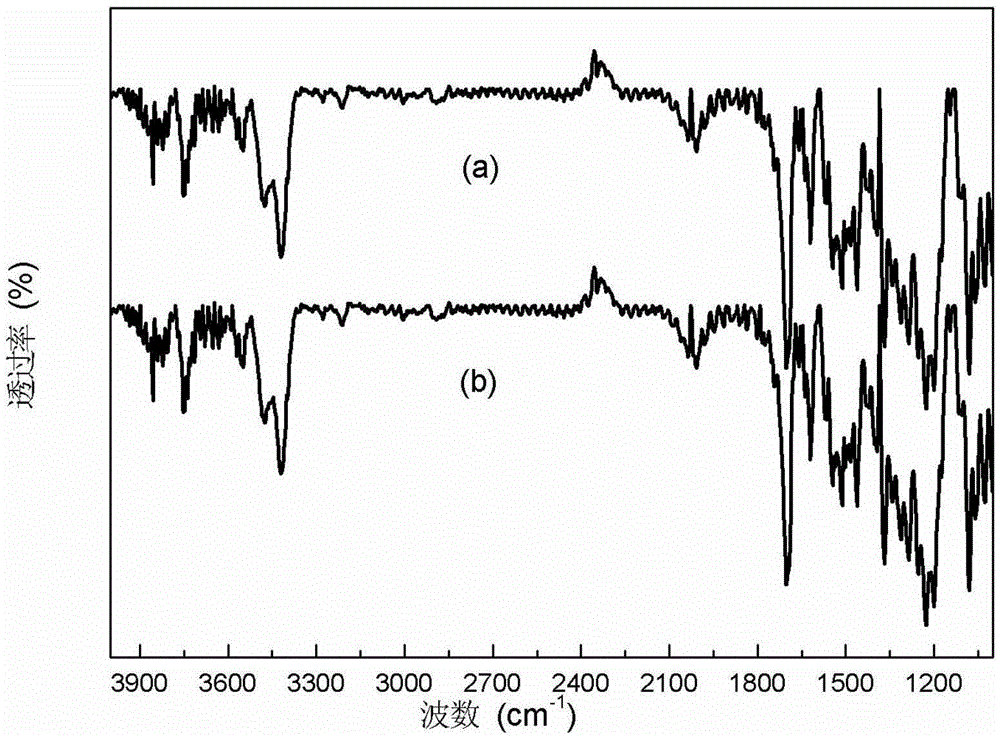
Image
Examples
Embodiment 1
[0035] Weigh 10g of dimethyl oxalate, 10g of urea and 10g of methanol and fill them into a 100ml reaction vessel that is airtight and pressure-resistant and equipped with a heating and stirring device, and feed N into the reactor. 2 Replace the air in the kettle for 3 consecutive replacements, and finally feed in N 2 Pressurize to 0.5MPa, set the temperature at 70°C, stir and heat to dissolve the raw materials completely and mix them evenly. The reaction temperature was set at 150° C., the stirring speed was set at 100 rpm, and the reaction was continued for 12 hours until the temperature in the reaction kettle dropped to room temperature. Open the tail gas venting needle valve to reduce the pressure in the reactor to 0.1 MPa. Open the reaction kettle, take out the reacted mixture, and place it in a flask of a rotary evaporator. Set the vacuum degree to 3mmHg, the temperature to 80°C, and the rotation speed to 100rpm. Distill under reduced pressure for 5 hours to collect 8.5...
Embodiment 2
[0037] Same as the embodiment of Example 1, the difference is that the reaction pressure is 0.7 MPa, and the amount of methanol added is 20 g. Product analysis results are shown in Table 1
Embodiment 3
[0039] Same as the embodiment of Example 1, the difference is that the reaction pressure is 0.7 MPa, and the amount of methanol added is 30 g. Product analysis results are shown in Table 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com