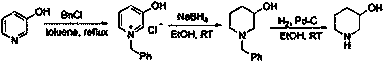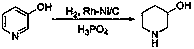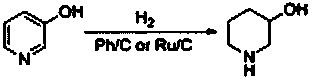Preparation method of 3-hydroxy piperidine
A technology of hydroxypiperidine and hydroxypyridine, which is applied in the field of synthesis of 3-hydroxypiperidine, can solve the problems of long synthesis steps, poor atom economy, harsh reaction conditions, etc., achieve stable yield, weaken passivation, and recycle high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 1 g of rhodium-nickel / carbon bimetallic catalyst (10% rhodium and 1% nickel), 10 g (0.11 mol) of 3-hydroxypyridine, 0.3 g (3.1 mmol) of phosphoric acid, and isopropanol (55 mL) As a solvent, put it into the reactor and pass hydrogen to the internal pressure of 3atm, 25 o C reacted for 3h, released hydrogen after the reaction was over, filtered the catalyst, concentrated the reaction solution under reduced pressure, and obtained crude product was distilled under reduced pressure (65-67 oC ,2mmHg) to get 10.7g of 3-hydroxypiperidine, yield: 96%; 1 HNMR (400MHz, CDCl 3 ):δ=3.56(m,1H),2.99(m,1H),2.80-2.39(m,3H),1.93(m,1H),1.74(m,1H),1.51-1.33(m,3H)ppm .
Embodiment 2
[0027] Weigh 500g of rhodium-nickel / carbon bimetallic catalyst (the rhodium content is 5%, the nickel content is 0.5%), 3-hydroxypyridine 10kg (105.3mol), phosphoric acid 0.5kg (5.1mol), water (42L) as solvent , put into the reactor and pass hydrogen to the internal pressure of 5atm, 50 o C reacted for 30min, released hydrogen after the reaction was over, filtered the catalyst, and the reaction liquid was distilled under reduced pressure (65-67 o C, 2mmHg) to obtain 9.78kg of 3-hydroxypiperidine, yield: 92%.
Embodiment 3
[0028] Embodiment 3 Catalyst recovery experiment
[0029] Weigh 100g of rhodium-nickel / carbon bimetallic catalyst (wherein rhodium content is 10%, nickel content is 1%), 3-hydroxypyridine 1kg (10.53mol), phosphoric acid 30g (0.31mol), water (5L) as solvent, Put hydrogen into the reactor to the internal pressure of 3atm, 50 o C reaction, release hydrogen after the reaction is over, filter out the catalyst and directly use it for the next catalytic cycle, and the reaction liquid is distilled under reduced pressure (65-67 oC , 2mmHg) to 3-hydroxypiperidine. Catalyst recycling 8 times product yield is as follows:
[0030] Cycles Reaction time (h) Product yield (%) 1 1 95 2 1 94.5 3 1 93.8 4 1.5 94.5 5 1.5 93.2 6 2 94 7 2 94.5 8 2 94
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com