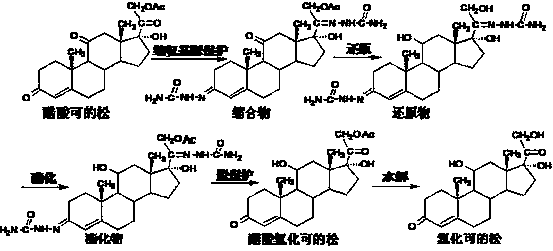
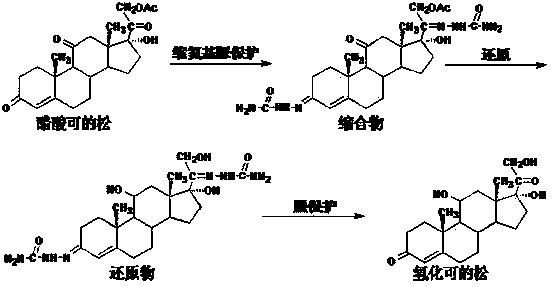
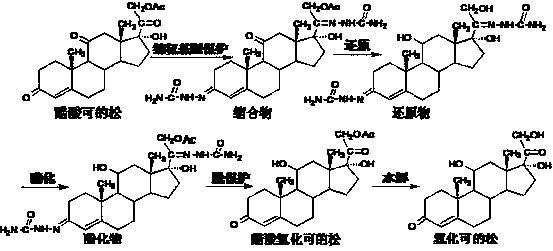
Method for preparing hydrocortisone
A technology of hydrocortisone and hydrocortisone acetate, applied in the directions of organic chemistry, steroids, etc., can solve the problem of inability to improve the primary mass yield of the crude product directly precipitated, time-consuming concentration and evaporation of the solvent, difficulty in crystallization of the product, etc. problems, to avoid the phenomenon of foam overflow, novel process route, and simplify the post-processing operation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 900ml of acetone, 30g of hydrocortisone intermediate reduction product, 12g of sodium acetate, 45ml of acetic anhydride, and 9ml of acetic acid to the reaction flask in sequence, and react at reflux at elevated temperature for 5 hours. TLC showed that the reaction was complete, and most of the solvent was recovered by distillation under normal pressure, and the solvent was evaporated under reduced pressure. The temperature was lowered to below 5°C. After stirring for 30 minutes, it was allowed to stand for more than 2 hours, filtered and dried to obtain 31.3 g of esterified compound.
[0040] Put 30g of the esterified product into a solution made up of 210ml of 36% hydrochloric acid, 390ml of water and 15ml of chloroform, and stir at 25℃~30℃ for 30 minutes to dissolve. The temperature is lowered, and a solution prepared by 15g sodium nitrite and 150ml water is added dropwise at 5℃~10℃. After the addition, continue to react at 5°C to 10°C for 2 hours. TLC shows that th...
Embodiment 2
[0043] 900ml of acetone, 30g of hydrocortisone intermediate reductant, 15g of potassium acetate, 51ml of acetic anhydride, and 9ml of acetic acid were added to the reaction flask in sequence, and the reaction was heated and refluxed for 6 hours. TLC showed that the reaction was complete, and most of the solvent was recovered by distillation under normal pressure, and the solvent was evaporated under reduced pressure. The temperature was lowered to below 5°C. After stirring for 30 minutes, it was allowed to stand for more than 2 hours, filtered and dried to obtain 31.5 g of esterified compound.
[0044] Put 30g of the esterified product into a solution made up of 210ml of 36% hydrochloric acid, 390ml of water and 15ml of chloroform, and stir at 25℃~30℃ for 30 minutes to dissolve. The temperature is lowered, and a solution prepared by 15g sodium nitrite and 150ml water is added dropwise at 5℃~10℃. After the addition, continue to react at 5°C to 10°C for 2 hours. TLC shows that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com