Solvent thermal preparation method of NiCo2O4 nano-material
A technology of nanomaterials and nickel cobaltate, which is applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of uneven distribution of nickel cobaltate particles, poor dispersion, low output, etc., and achieve a suitable large-scale The production and preparation methods are simple and the product cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
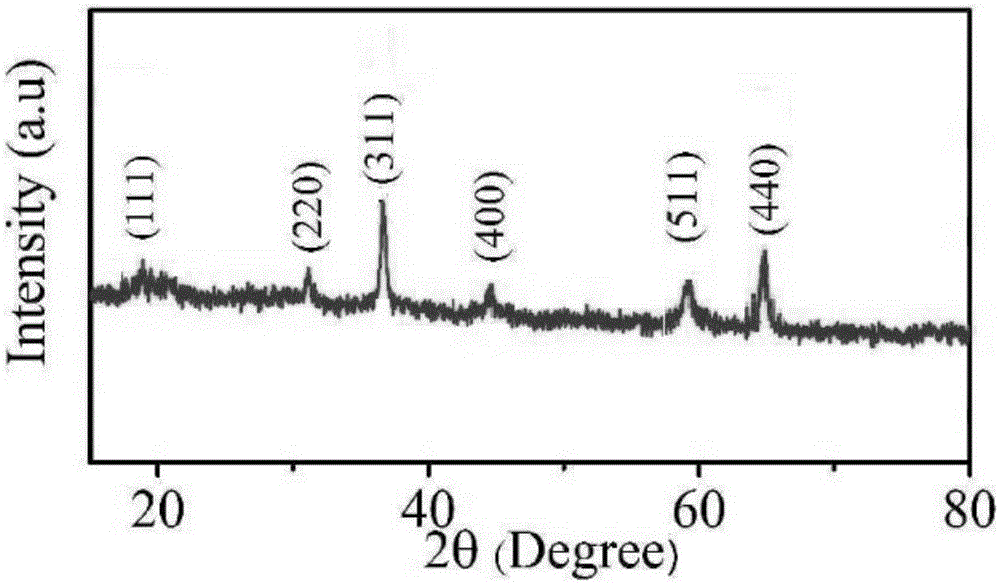
Image
Examples
Embodiment 1
[0025] Solvothermal method of the present invention prepares the method step of nickel cobaltate nanometer material as follows:
[0026] (1) Weigh 2.9gNi(NO 3 ) 2 ·6H 2 O (contains 0.01mol of nickel ions), 5.8gCo(NO 3 ) 2 ·6H 2 O (containing cobalt ion 0.02mol) and 0.085gNaNO 3 Disperse in 50mL of polyethylene glycol 400, then add 10g of urea, sonicate until completely dissolved, then transfer the resulting mixed solution to a reaction kettle, heat to 200°C, and react at a constant temperature for 16h under stirring conditions, and the stirring speed is 270r / min, using non-magnetic stirring; after the reaction, the precursor solution was cooled to room temperature, and the obtained product was washed 3 times with deionized water, then washed 3 times with absolute ethanol, and dried at a constant temperature at 80°C. That is, the precursor is obtained.
[0027] (2) The obtained precursor was placed in a tube furnace and heat-treated at a constant temperature of 390° C. ...
Embodiment 2
[0030] Solvothermal method of the present invention prepares the method step of nickel cobaltate nanometer material as follows:
[0031] (1) Weigh 2.9gNi(NO 3 ) 2 ·6H 2 O (contains 0.01mol of nickel ions), 5.8gCo(NO 3 ) 2 ·6H 2 O (containing cobalt ion 0.02mol) and 0.085gNaNO 3 Disperse in 45mL polyethylene glycol 400, then add 12g urea, sonicate until completely dissolved, then transfer the resulting mixed solution to a reaction kettle, heat to 180°C, and react at a constant temperature for 20h under stirring conditions, and the stirring speed is 260r / min, using non-magnetic stirring; after the reaction, the precursor solution was cooled to room temperature, and the obtained product was washed 3 times with deionized water, then washed 3 times with absolute ethanol, and dried at a constant temperature at 75°C. That is, the precursor is obtained.
[0032] (2) The obtained precursor was placed in a tube furnace and heat-treated at a constant temperature of 400° C. for 2 ...
Embodiment 3
[0035] Solvothermal method of the present invention prepares the method step of nickel cobaltate nanometer material as follows:
[0036] (1) Weigh 2.9gNi(NO 3 ) 2 ·6H 2 O (contains 0.01mol of nickel ions), 5.8gCo(NO 3 )2 ·6H 2 O (containing cobalt ion 0.02mol) and 0.085gNaNO 3 Disperse in 40mL polyethylene glycol 400, then add 15g urea, sonicate until completely dissolved, then transfer the resulting mixed solution to a reaction kettle, heat to 180°C, and react at a constant temperature for 24h under stirring conditions, and the stirring speed is 280r / min, using non-magnetic stirring; after the reaction, the precursor solution was cooled to room temperature, and the obtained product was washed with deionized water for 3 times, and then washed with absolute ethanol for 3 times, and dried at a constant temperature at 70°C. That is, the precursor is obtained.
[0037] (2) The obtained precursor was placed in a tube furnace and heat-treated at a constant temperature of 410°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com