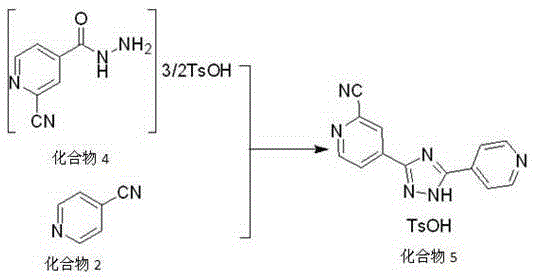
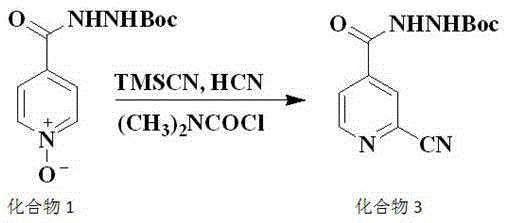
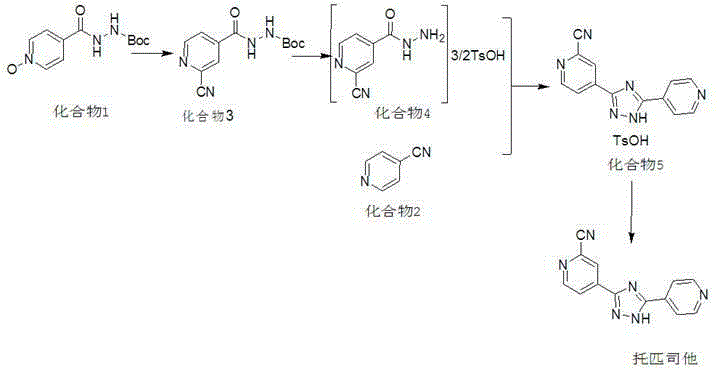
Preparation method of topiroxostat
A technology for topicastat and compounds, which is applied in the fields of drug synthesis and organic compound synthesis and preparation, can solve the problems of rare starting materials, low purity, and difficulty in obtaining starting materials, and achieves simple and feasible process route and reasonable method selection. , the effect of overcoming the problems of the prior art
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0024] The main process involved in this embodiment is the same as in the summary of the invention, wherein the specific process of compound 3 and compound 5 is as follows:
[0025]
[0026] 1. Preparation of compound 3:
[0027] At room temperature, add 3.1Kg of compound 1 and 3.95Kg of dimethylcarbamoyl chloride into 20L of acetonitrile, and add 1.822Kg of trimethylsilyl cyanide under nitrogen protection; heat up to 60°C for 3 hours; after the reaction is complete, reduce the reaction solution to After room temperature, pour into 200L water and stir for crystallization for 3 hours, and adjust the pH value to 6-8; after the crystallization is complete, filter, wash the filter cake with 500mL water, and dry at 45°C to obtain 1.55Kg of off-white powder, which is compound 3. The rate is 48%.
[0028] 2. Preparation of compound 3:
[0029] At room temperature, add 4Kg of compound 1 and 5.1Kg of dimethylcarbamoyl chloride into 20L of acetonitrile, and add 2.35Kg of trimethyls...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com