Preparation method and application of phosphorescence iridium complexes with mitochondrial targeting function
A phosphorescent iridium complex, iridium complex technology, applied in indium organic compounds, platinum group organic compounds, compounds containing elements of Group 8/9/10/18 of the periodic table, etc., can solve the complex and unfavorable probe structure. Synthesis and preparation, etc., to achieve the effect of improving signal-to-noise ratio, good biocompatibility, and deep tissue penetration depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of auxiliary ligand containing triphenylphosphorus
[0026]
[0027] Preparation of compound 2: compound 1 (26mmol) and sodium methoxide solution were stirred and reacted at room temperature for 22 hours, ammonium chloride (28mmol) was added and the reaction continued for 6 hours, filtered to remove unreacted ammonium chloride, and the filtrate was spin-dried to remove the methanol solvent , A light yellow solid powder was obtained, and then washed three times with ether to remove the unreacted compound 1, and then recrystallized using a mixed solvent of ethanol and ether to obtain a white product compound 2. Yield: 83%. 1 HNMR(400MHz,DMSO)δ=9.69(s,4H),8.85-8.73(m,1H), 8.45(d,J=8.0Hz,1H), 8.14(td,J=7.8,1.7Hz,1H), 7.85–7.71(m,1H). 13CNMR (101MHz, DMSO) δ=162.67, 150.33, 144.37, 138.76, 129.01, 124.00.
[0028] Serial number
[0029] Preparation of compound 3: under the catalysis of sodium ethoxide, compound 2 (5mmol) and ethyl acetoacetate (5mmol) ...
Embodiment 2
[0036] Example 2: Ir-P(Ph) containing triphenylphosphoric iridium complex 3 (7) Preparation
[0037]
[0038] Compound Ir-P(Ph) 3 (7) Preparation: Compound 5 (0.14mmol) and iridium dichloride bridge (0.06mmol) were stirred and refluxed overnight at 55°C in a mixed solvent of dichloromethane (3mL) and methanol (1mL) under nitrogen atmosphere. Cool the reaction solution to room temperature and add KPF to it 6 (1.4mmol) continue to stir for 4 hours. After the reaction, the organic solvent is removed by rotary evaporation, and the obtained solid is purified by column to obtain a red powdery solid Ir-P(Ph) 3 (7). The yield was 36%. 1 HNMR(400MHz,DMSO)δ8.43(d,J=8.1Hz,1H),8.11(dt,J=10.7,5.4Hz,3H),8.03-7.95(m,2H),7.93-7.70(m,22H ), 7.36–7.28(m,2H), 7.23–6.97(m,14H), 6.96–6.88(m,3H), 6.86–6.70(m,9H), 6.52(dd,J=6.9,4.4Hz,2H ), 4.37 (s, 2H), 3.56 (s, 2H), 1.99 (s, 3H), 1.50 (dd, J = 60.1, 31.4 Hz, 8H).
[0039] Serial number
Embodiment 3
[0040] Example 3: Ir-P(Ph) complex of iridium 3 (7) Absorption and emission spectrum test
[0041] The spectrum test concentration adopted in the present invention is 10 μM, the test solvent is a PBS solution mixed with 1% DMSO, and when the emission spectrum is measured, the excitation wavelength is 405 nm.
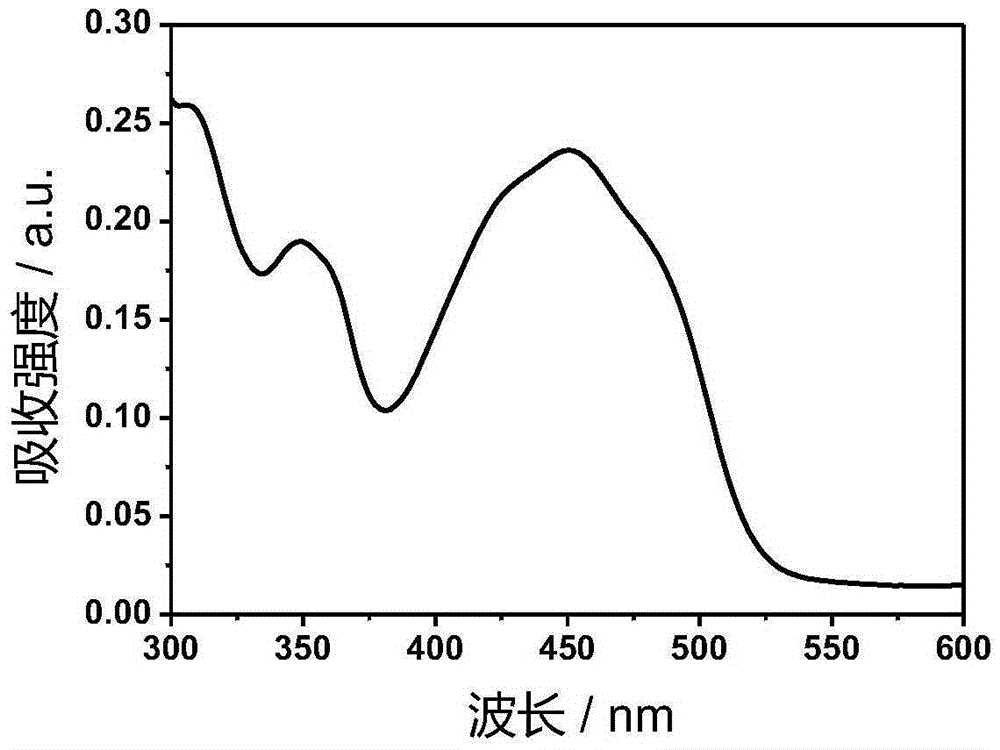
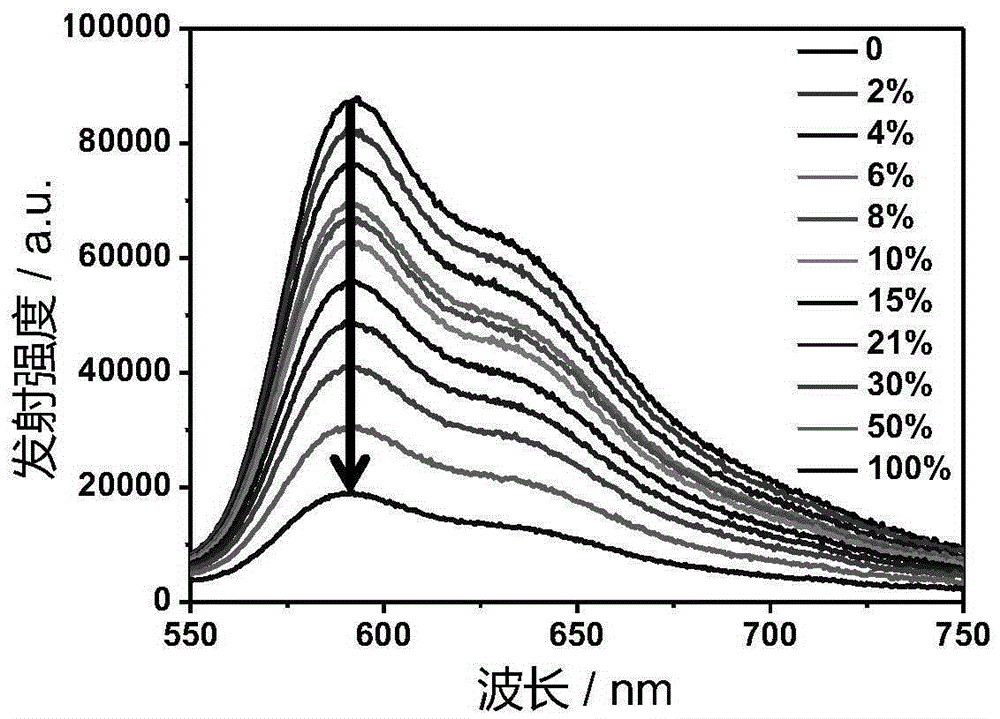
[0042] Ir-P(ph) 3 The absorption and emission spectra such as figure 1 with figure 2 Shown. The complex exhibits strong absorption in the ultraviolet region of 250-380nm and the visible blue region of 400-500nm. In particular, the complex can be excited by visible light, which greatly reduces the damage of the excitation light source to cells when doing cell imaging experiments. Its emission is relatively wide, and the emission peak is at 602nm. The red light emission increases the penetration depth of biological tissues, making it more suitable for biological imaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com