Chiral binuclear Ir (III) metal-organic double helix structure compound and preparation method thereof
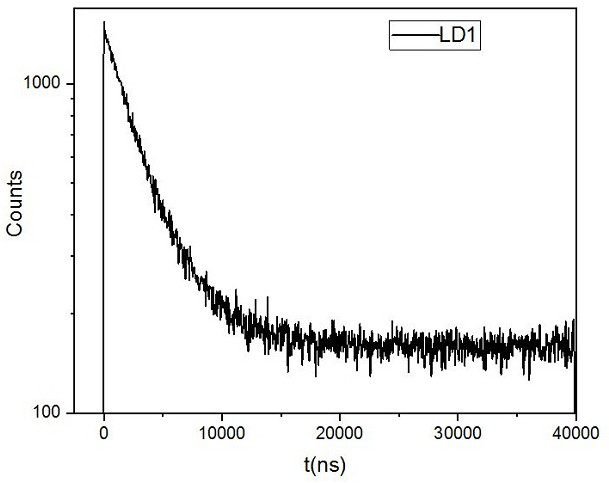
A double helix structure and compound technology, applied in the direction of indium organic compounds, organic chemical methods, platinum group organic compounds, etc., can solve the problems of difficult to maintain chiral structure and low chemical stability, and achieve good biocompatibility, phosphorescence The effect of long emission life and strong binding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
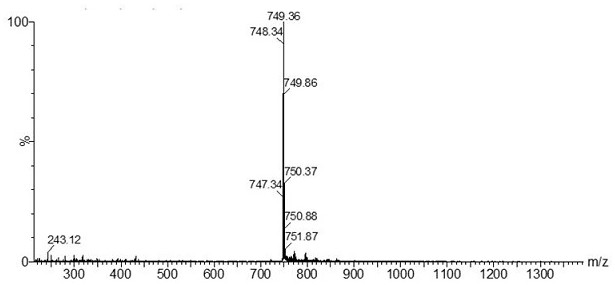
[0047] 1) Take the ligand 4-(2-pyridyl)-benzaldehyde (916 mg, 5 mmol), IrCl 3 • xH 2 O (600 mg, 2 mmol) was placed in a 50 mL two-necked flask, 30 mL of mixed solvent ethylene glycol methyl ether / water (3 / 1, V / V) was added, and argon was introduced for 30 minutes to exhaust the air in the system Afterwards, the temperature was raised to 120 °C and stirred for 24 h in the dark. After cooling to room temperature, the filter cake was obtained by suction filtration, and the filter cake was washed with water, ethanol and ether respectively to obtain the red dichloro-bridged complex [Ir(L-CHO) 2 Cl] 2 .
[0048] 2) Weigh [Ir(L-CHO) 2 Cl] 2 (300 mg, 0.25 mmol) into a 250 mL three-necked flask, dissolved in 100 mL of dry dichloromethane / methanol (4 / 1, V / V) mixed solvent, then added sodium methoxide (40.50 mg, 0.75 mmol), and ventilated After 30 minutes, exhaust the air in the system and add L-proline (L-Pro) (86.40 mg, 0.75 mmol), heat up to 50 °C and reflux, and react in the da...
Embodiment 2
[0051] Take L1-a (156.80 mg, 0.20 mmol) and dissolve it in 120 mL of dry dichloromethane solution, blow in argon, exhaust the air in the system, add the ligand dipyrido[3,2-a:2',3' -c]phenazine (dppz, 84.70 mg, 0.30mmol), after stirring at room temperature in the dark for 10 h, the solvent was removed by rotary evaporation under reduced pressure, and separated by silica gel column chromatography to obtain a yellow solid L1-b. The nuclear magnetic spectrum of the obtained compound L1-b is as figure 2 shown.
Embodiment 3
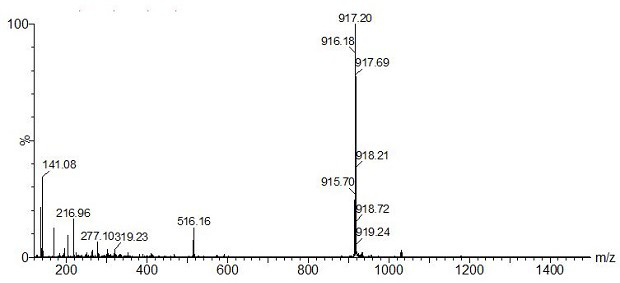
[0053] 1) Weigh the intermediate product [Ir(L-CHO) of Example 1 2 Cl] 2 (300.0 mg, 0.25 mmol) into a 250 mL three-necked flask, dissolved in 100 mL of dry dichloromethane / methanol (4 / 1, V / V) mixed solvent, then added sodium methoxide (40.5 mg, 0.75 mmol), and ventilated After 30 min, exhaust the air in the system, add D-proline (D-Pro) (86.4 mg, 0.75 mmol), heat up to 50 °C and reflux, and react in the dark for 12 h, cool to room temperature, and then add water, dichloro Extracted with methane and saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated to remove solvent, separated by silica gel column chromatography to obtain red solid Ir(L-CHO) 2 (D-Pro).
[0054] 2) Take Ir(L-CHO) 2 (D-Pro) (40.40 mg, 0.06 mmol) was dissolved in 30 mL of dry acetonitrile solution, argon gas was introduced to exhaust the air in the system, trifluoroacetic acid (9 μL, 0.12 mmol) was slowly added, and stirred at room temperature in the dark After 4 h, ammonium hexafluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com