Complex formed from aromatic nitrile compound polymerization product and sulfur, preparation method and uses thereof
A composite and compound technology, applied in the field of electrochemistry, can solve the problem of lithium-sulfur battery cathode material without the preparation method of aromatic nitrile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] This example is used to illustrate the preparation method of the material "polymer / sulfur" provided by the present invention and its preparation and application method as a cathode material for a lithium-sulfur battery.
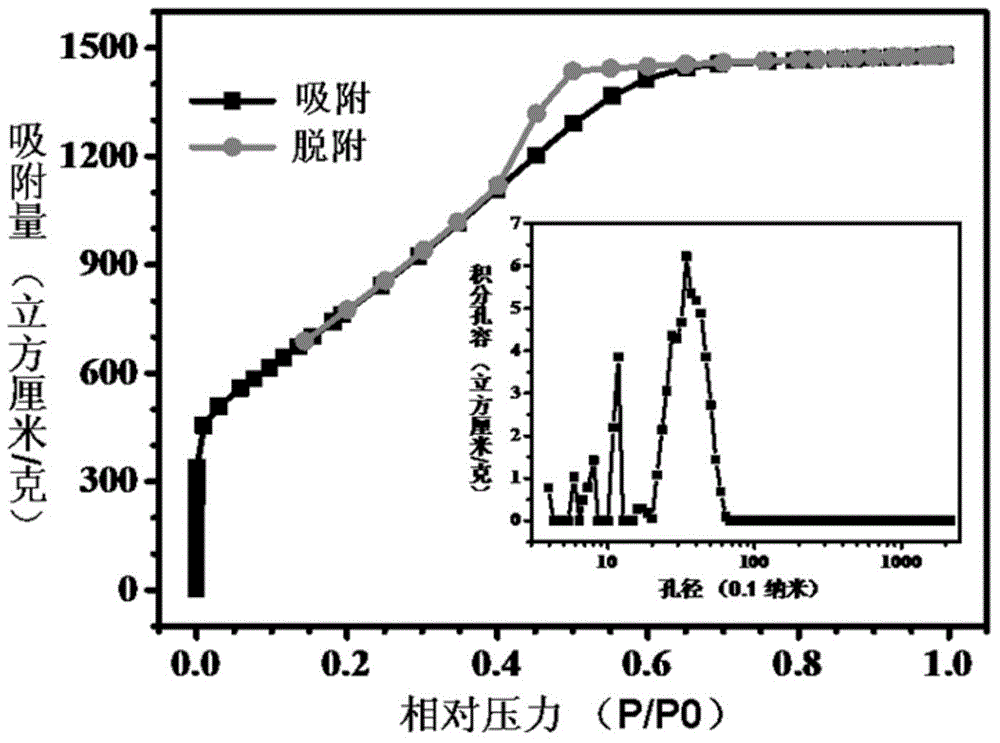
[0068]Mix 1g (7.81mmol) of terephthalonitrile (CAS No. 623-26-7) and 0.53g (3.9mmol) of anhydrous zinc chloride, and transfer them to a 10ml quartz tube, and replace the quartz with argon air in the tube, seal it and put it into a muffle furnace, react at 700°C for 0.5 hours, cool down to room temperature (25°C) naturally, open the glass tube, take out the polymerization product, and successively use 1mol / L Washed with hydrochloric acid and pure water, then placed in a vacuum oven and dried at 120°C for 10 hours to obtain an N-doped polymer product, which can be used as an oxygen reduction catalyst. Among them, the content of nitrogen in the catalyst is 10.3%, and the specific surface area is 1710m 2 / g, the pore size distribution range is: 0.5~4nm. ...
Embodiment 2
[0072] This example is used to illustrate the preparation method of the material "polymer / sulfur" provided by the present invention and its preparation and application method as a cathode material for a lithium-sulfur battery.
[0073] Mix 1 g (7.81 mmol) of terephthalonitrile (CAS number: 623-26-7) and 10.64 g (78 mmol) of anhydrous zinc chloride, and transfer them to a 10ml quartz tube, and replace the quartz tube with argon After sealing it, put it into a muffle furnace, react at 700°C for 80 hours, cool it to room temperature (25°C) naturally, open the glass tube, take out the polymerized product, and successively wash it with 1mol / L hydrochloric acid , washed with pure water, and then placed in a vacuum drying oven and dried at 120 °C for 10 hours to obtain an N-doped polymer product, which can be used as an oxygen reduction catalyst. In the obtained oxygen reduction catalyst, the content of nitrogen element was 2.1%. The specific surface area is 2980m 2 / g, pore size d...
Embodiment 3
[0077] This example is used to illustrate the preparation method of the material "polymer / sulfur" provided by the present invention and its preparation and application method as a cathode material for a lithium-sulfur battery.
[0078] Mix 1g (7.81mmol) of terephthalonitrile (CAS No.: 623-26-7) and 1.06g (7.8mmol) of anhydrous zinc chloride, and transfer them to a 10ml glass tube, replace the glass with argon air in the tube, seal it and put it into a muffle furnace, react at 300°C for 5 hours, cool down to room temperature (25°C) naturally, open the glass tube, take out the polymerized product, and use 1mol / L of Washed with hydrochloric acid and pure water, then placed in a vacuum oven and dried at 120°C for 10 hours to obtain an N-doped polymer product, which can be used as an oxygen reduction catalyst. Among them, the content of nitrogen in the catalyst is 18.4%, and the specific surface area is 143m 2 / g, the pore size distribution range is: 0.4~2.8nm.
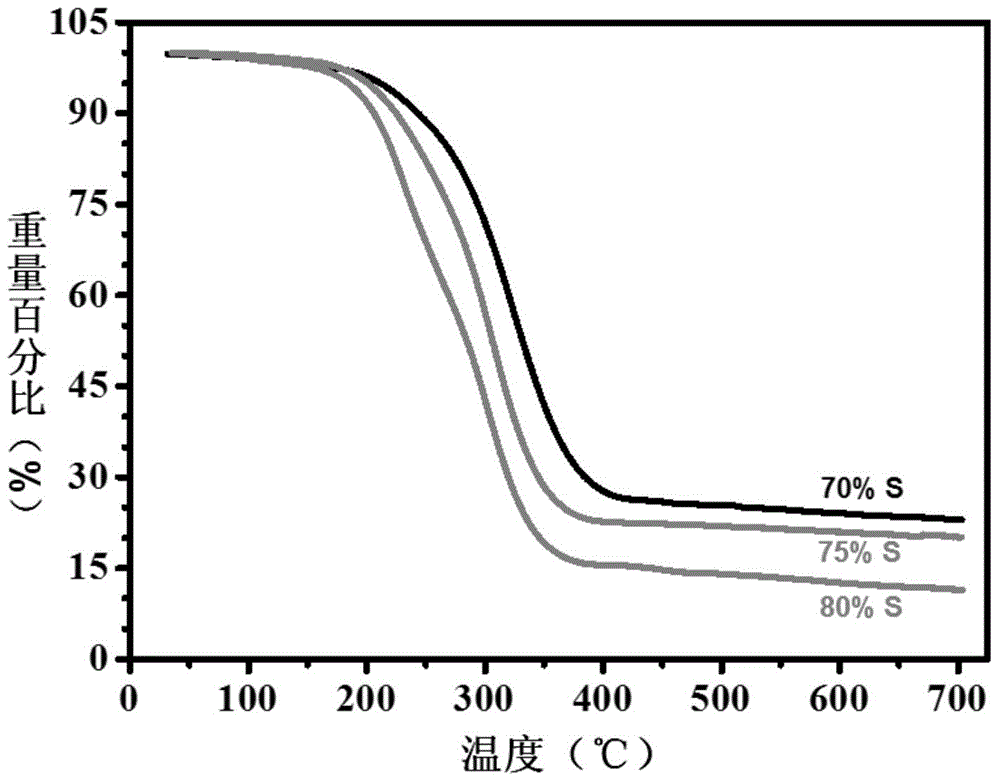
[0079] Mix 500mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size distribution | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com