Preparation method for all-solid-state lithium ion electrolyte material Li7La3Zr2O12
An electrolyte material, lithium ion technology, applied in electrolytes, circuits, electrical components, etc., can solve the problems of easy mixing of molten salt into powder, reduce battery manufacturing costs, and require high-temperature sintering, so as to speed up ion transfer and reduce The effect of production cost and short calcination time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
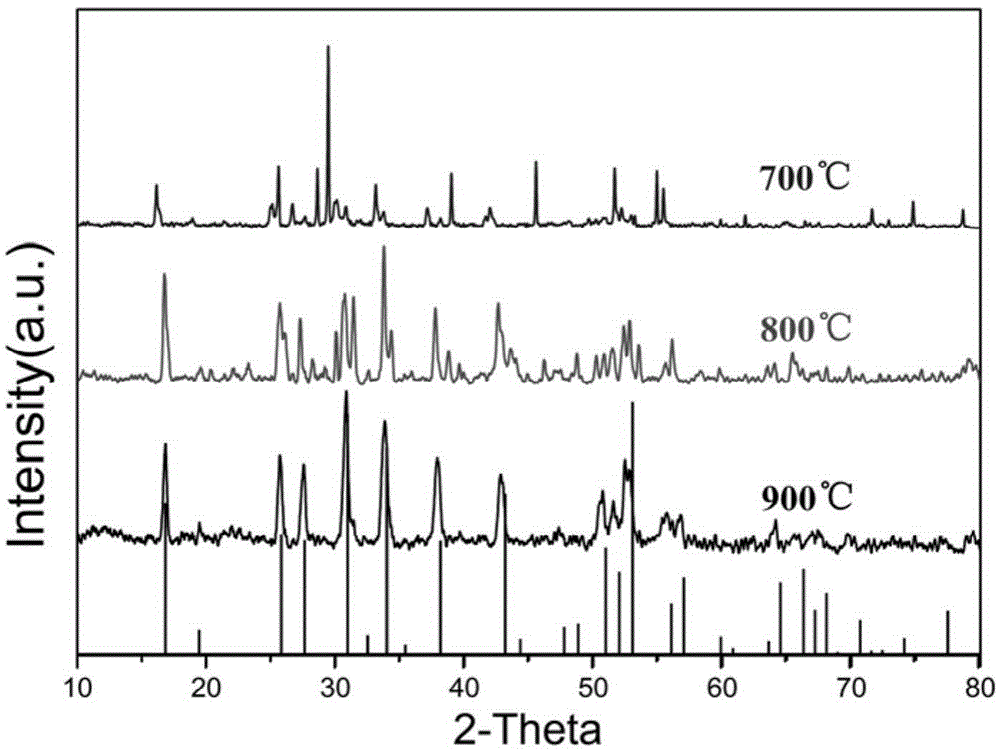
[0042] First, weigh lithium nitrate LiNO 3 10.33g, lithium hydroxide LiOH6.27g, dilanthanum trioxide La 2 o 3 3.66g, zirconium dioxide ZrO 2 1.83g. Then pour it into an agate mortar and grind it thoroughly for 15 minutes to mix the raw materials evenly, and then transfer the ground powder to a magnesia crucible. The crucible was placed in a tube furnace, and high-purity argon gas was introduced slowly for 10 minutes to remove the air in the tube. Then ignited and calcined, the furnace temperature was raised to 900°C after 60 minutes, and calcined at this temperature for 5 hours. After the calcination, it was naturally lowered to room temperature in an argon atmosphere. Finally, the product was taken out, washed three times by centrifugation with deionized water, and dried under vacuum at 120° C. for 12 hours.
Embodiment 2
[0044] First, weigh lithium nitrate LiNO 3 6.89g, lithium hydroxide LiOH4.18g, dilanthanum trioxide La 2 o 3 2.44g, zirconium dioxide ZrO 2 1.22g. Then pour it into an agate mortar and grind it thoroughly for 15 minutes to mix the raw materials evenly, and then transfer the ground powder to a magnesia crucible. The crucible was placed in a tube furnace, and high-purity argon gas was introduced slowly for 10 minutes to remove the air in the tube. Then ignited and calcined, the furnace temperature was raised to 800°C after 60 minutes, and calcined at this temperature for 5 hours. After the calcination, it was naturally lowered to room temperature in an argon atmosphere. Finally, the product was taken out, washed three times by centrifugation with deionized water, and dried under vacuum at 120° C. for 12 hours.
Embodiment 3
[0046] First, weigh lithium nitrate LiNO 3 6.89g, lithium hydroxide LiOH4.18g, dilanthanum trioxide La 2 o 3 2.44g, zirconium dioxide ZrO 2 1.22g. Then pour it into an agate mortar and grind it thoroughly for 15 minutes to mix the raw materials evenly, and then transfer the ground powder to a magnesia crucible. The crucible was placed in a tube furnace, and high-purity argon gas was introduced slowly for 10 minutes to remove the air in the tube. Then ignite and calcinate, the furnace temperature rises to 700°C after 60 minutes, and calcines at this temperature for 5 hours. After the calcination, it was naturally lowered to room temperature in an argon atmosphere. Finally, the product was taken out, washed three times by centrifugation with deionized water, and dried under vacuum at 80° C. for 12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com