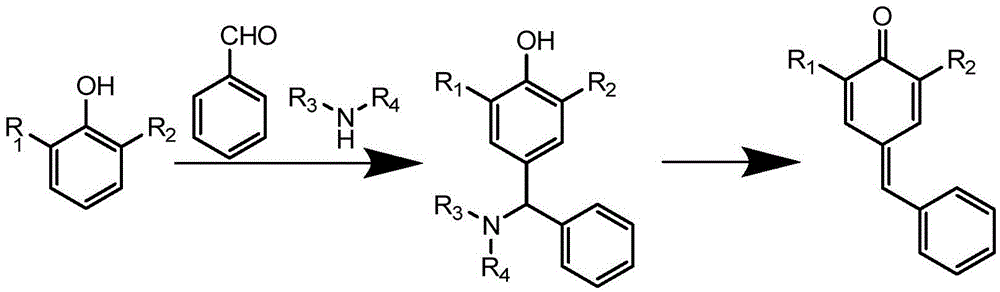
One-pot synthesis method for 4-aryl methylene-2,6-disubstituted-2,5-cyclohexadiene-1-one
A technology of aryl methylene and cyclohexadiene, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, carbon-based compounds, etc., can solve the difficult operation of the format reaction, is difficult to obtain, and cannot meet the needs of the pharmaceutical field Questions such as requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Add 242.8g (1.1768mol) of 2,6-di-tert-butylphenol, 128.4g (1.2099mol) of benzaldehyde, 29.2g of hexahydropyridine (84.0g will be added dropwise later, totaling 113.2g, 1.3294mol), toluene into a 1000ml three-necked flask 35ml, heat up and reflux to divide the water, add dropwise 84.0g of hexahydropyridine, after the addition is complete, then reflux to divide the water for 3h;
[0057] Then add acetic anhydride (1.43mol), 110-125 ℃ insulation reaction for 30min, add toluene, wash with water, wash with saturated saline, dry over anhydrous sodium sulfate, filter, desolvate under reduced pressure, mixed solvent (ethyl acetate: n-hexane volume ratio =3:1) recrystallized to obtain 314.2g of wet product, and dried to obtain 274.2g (yield: 79.1%).
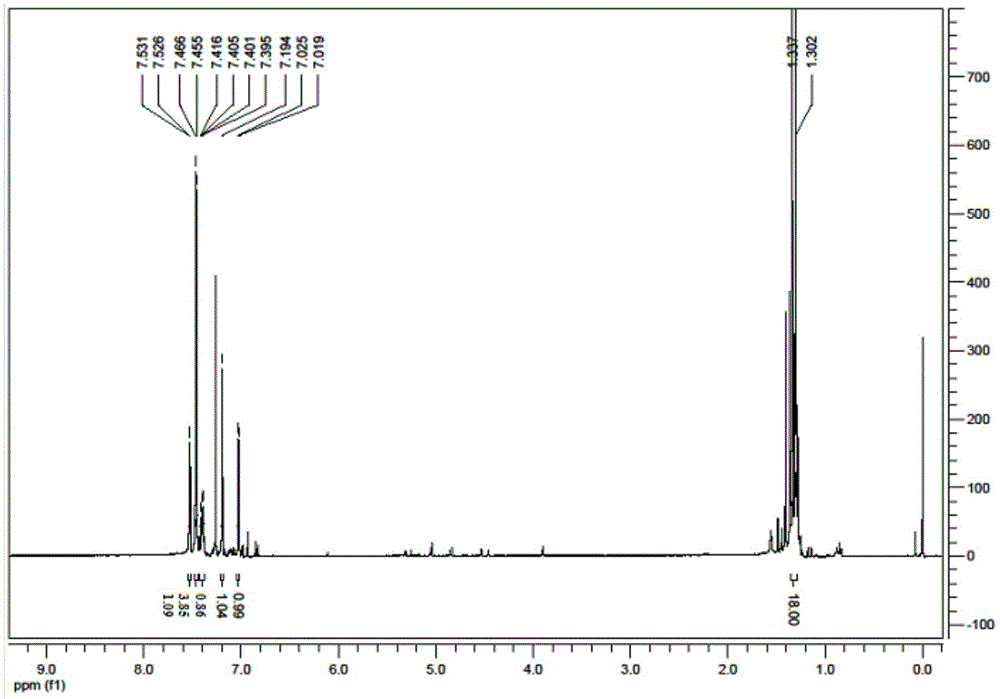
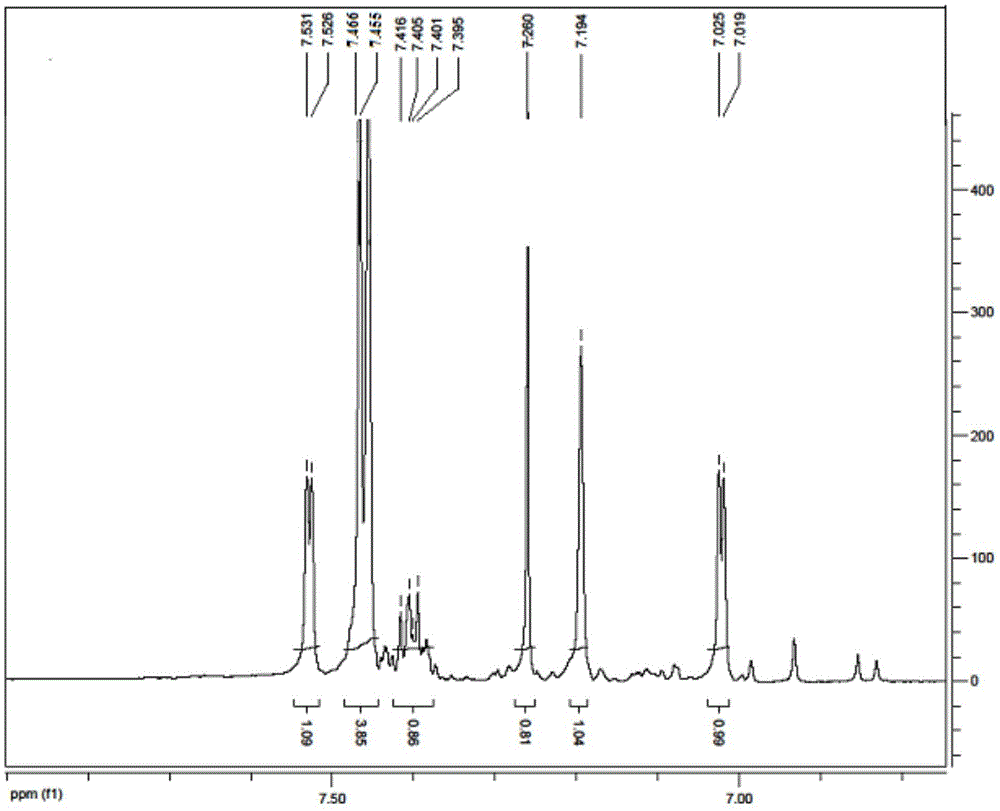
[0058] This embodiment makes the product 1 HNMR picture as shown figure 2 As shown, the displacement values (ppm) in the upper part of the figure are from left to right: 7.531, 7.526, 7.466, 7.455, 7.416, 7.405, 7.401, 7.395, ...
Embodiment 2
[0064] Add 60.7g (0.2942mol) of 2,6-di-tert-butylphenol, 32.1g (0.3025mol) of benzaldehyde, 7.3g of hexahydropyridine (21.0g will be added dropwise later, totaling 28.3g, 0.3324mol), toluene into a 250ml three-necked flask 9ml, heat up and reflux to divide the water, add dropwise 21.0g of hexahydropyridine, after the addition is complete, then reflux to divide the water for 3h;
[0065] Then add propionic anhydride (0.3573mol), keep the reaction at 110-125°C for 30min, add toluene, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, precipitate under reduced pressure, ethyl acetate / n-hexane (3:1) The mixed solvent was recrystallized to obtain 80.8g of wet product, and dried to obtain 57.1g (yield: 65.9%). of the resulting product 1 HNMR chart and GC chart are similar to Example 1.
Embodiment 3
[0067] Add 20.8g (0.1008mol) of 2,6-di-tert-butylphenol into a 100ml three-necked flask, 11.0g (0.1036mol) of benzaldehyde, 2.5g of di-n-propylamine (9.0g will be added dropwise later, totaling 11.5g, 0.1138mol), toluene 3ml, heat up and reflux to divide the water, add 9.0g of di-n-propylamine dropwise, after the addition is completed, then reflux and divide the water for 3h;
[0068] Then add butyric anhydride (0.12mol), keep warm at 110-125°C for 30 minutes, add toluene, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and precipitate under reduced pressure, ethyl acetate / n-hexane (3:1) The mixed solvent was recrystallized to obtain 23.6g of wet product, and dried to obtain 20.8g (yield: 70.0%). of the resulting product 1 HNMR chart and GC chart are similar to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com