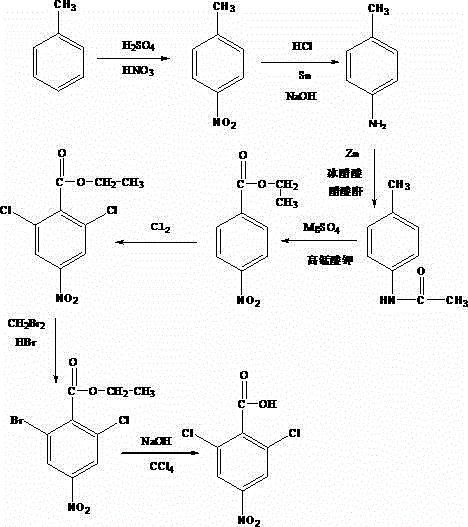
Synthesis method of 2-chloro-6-bromo-p-nitrobenzoic acid
A technology of p-nitrobenzoic acid and ethyl nitrobenzoate, applied in the field of synthesis of 2-chloro-6-bromo p-nitrobenzoic acid, can solve problems such as expensive formic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] First, 80 mL of toluene was added to a 500 mL three-necked flask, and 5 mL of sulfuric acid with a mass fraction of 90% and 8 mL of nitric acid with a mass fraction of 65% were slowly added thereto, and the reaction temperature was controlled at 30°C for 20 minutes to obtain p-nitrotoluene. Raise the temperature to 70°C, add 2mL of 20% hydrochloric acid solution dropwise into the three-necked flask, add 0.2g of tin powder and 5mL of 40% sodium hydroxide solution after the dropwise addition, stir evenly and react for 20min. To obtain p-toluidine; add 50mL of water, 0.1g of zinc powder, zeolite and 30mL of glacial acetic acid to the obtained p-toluidine, stir evenly, heat in a water bath to 100°C, and react for 40min, add 5mL of acetic anhydride, and the When the white mist stops adding heating, slowly pour the reactant in the beaker into 70mL of cold water under stirring while it is hot, and keep stirring, then perform suction filtration to obtain p-methylacetanilide; add...
example 2
[0021]First, take 90mL of toluene and add it to a 500mL three-neck flask, slowly add 5.5mL of sulfuric acid with a mass fraction of 92% and 9mL of nitric acid with a mass fraction of 68% respectively, and control the reaction temperature at 35°C for 25 minutes to obtain p-nitrotoluene , raise the temperature to 75°C, add 2.5mL hydrochloric acid solution with a mass fraction of 25% to the three-necked flask dropwise, add 0.3g tin powder and 5.5mL sodium hydroxide solution with a mass fraction of 45% after the dropwise addition, and stir to react for 25min , to obtain p-toluidine; add 55mL of water, 0.15g of zinc powder, zeolite and 40mL of glacial acetic acid to the obtained p-toluidine, stir well and then heat it in a water bath to 103°C. After reacting for 45min, add 8mL of acetic anhydride, and When white mist appears in the beaker, stop adding heating, slowly pour the reactant into 75mL of cold water under stirring in the beaker while it is hot, and keep stirring, then carry...
example 3
[0023] First, 100 mL of toluene was added to a 500 mL three-necked flask, and 6 mL of sulfuric acid with a mass fraction of 94% and 10 mL of nitric acid with a mass fraction of 70% were slowly added thereto, and the reaction temperature was controlled at 40°C for 30 minutes to obtain p-nitrotoluene. Raise the temperature to 80°C, drop 3mL of 30% hydrochloric acid solution into the three-necked flask with a straw, add 0.4g of tin powder and 6mL of 50% sodium hydroxide solution after the dropwise addition, stir evenly and react for 30min. To obtain p-toluidine; add 60mL of water, 0.2g of zinc powder, zeolite and 50mL of glacial acetic acid to the obtained p-toluidine, stir evenly and heat it in a water bath to 105°C. After reacting for 50min, add 10mL of acetic anhydride, and the When the white mist stops adding heating, slowly pour the reactant into 80mL of cold water under stirring in the beaker while it is hot, and keep stirring, then carry out suction filtration to obtain p-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com