Metaxalone preparations
A technology of metaxalone and dosage form, applied in the field of metaxalone preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0076] (1) Surfactants and polymers, including but not limited to polyethylene glycol, polyvinylpyrrolidone (PVP), polyvinyl alcohol, crospovidone, polyvinylpyrrolidone-polyvinyl acrylate copolymer, cellulose derivatives , hydroxypropylmethylcellulose, hydroxypropylcellulose, carboxymethylethylcellulose, hydroxypropylmethylcellulose phthalate, polyacrylates and polymethacrylates, urea, sugar polyols, and their polymers, emulsifiers, sugar gums, starches, organic acids and their salts, vinylpyrrolidone and vinyl acetate; and / or
[0077] (2) Binders such as various celluloses and cross-linked polyvinylpyrrolidone, microcrystalline cellulose; and / or
[0078] (3) fillers such as lactose monohydrate, anhydrous lactose, microcrystalline cellulose, and various starches; and / or
[0079] (4) Lubricants such as agents that act on the fluidity of the powder to be compressed, including colloidal silicon dioxide, talc, stearic acid, magnesium stearate, calcium stearate, silica gel; and / or...
Embodiment 1
[0110] Embodiment 1: the dry grinding of metaxalone
[0111] Chemically, metaxalone is 5-[3,5-dimethylphenoxy)methyl]-2-oxazolidinone. The empirical formula is C12H15NO3, which corresponds to a molecular weight of 221.25 g / mol. Metaxalone is a white to almost white, odorless crystalline powder, readily soluble in chloroform, soluble in methanol and 96% ethanol, but practically insoluble in ether or water. The mechanism of action of metaxalone in humans has not been established, but may be due to general central nervous system depression.
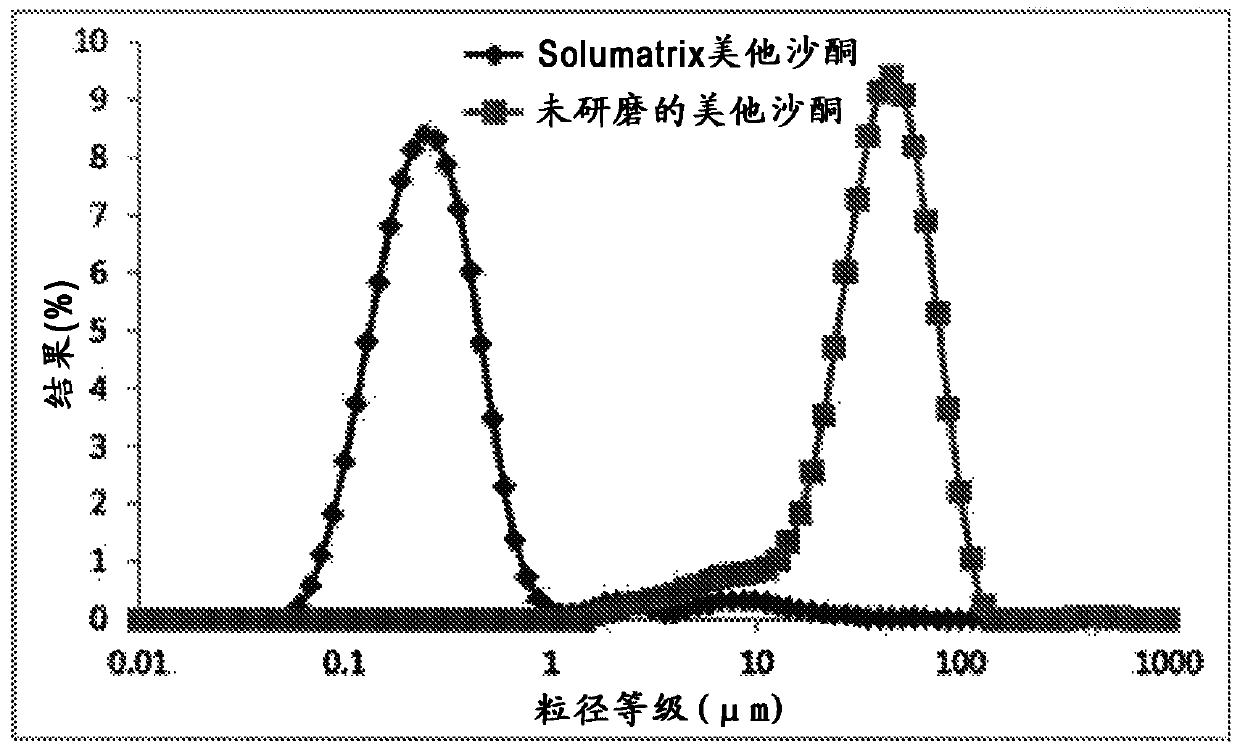
[0112] Submicron sized metaxalone drug substance particles were prepared by dry milling metaxalone drug substance (40%) with lactose monohydrate and sodium lauryl sulfate in an attritor mill containing stainless steel grinding media. The total batch size is approximately 1 kg. The ground powder is discharged to the bottom of the mill and collected for analysis and further processing. The particle size distribution of the milled metaxalon...
Embodiment 2
[0116] Embodiment 2: the preparation and characterization of submicron metaxalone tablet
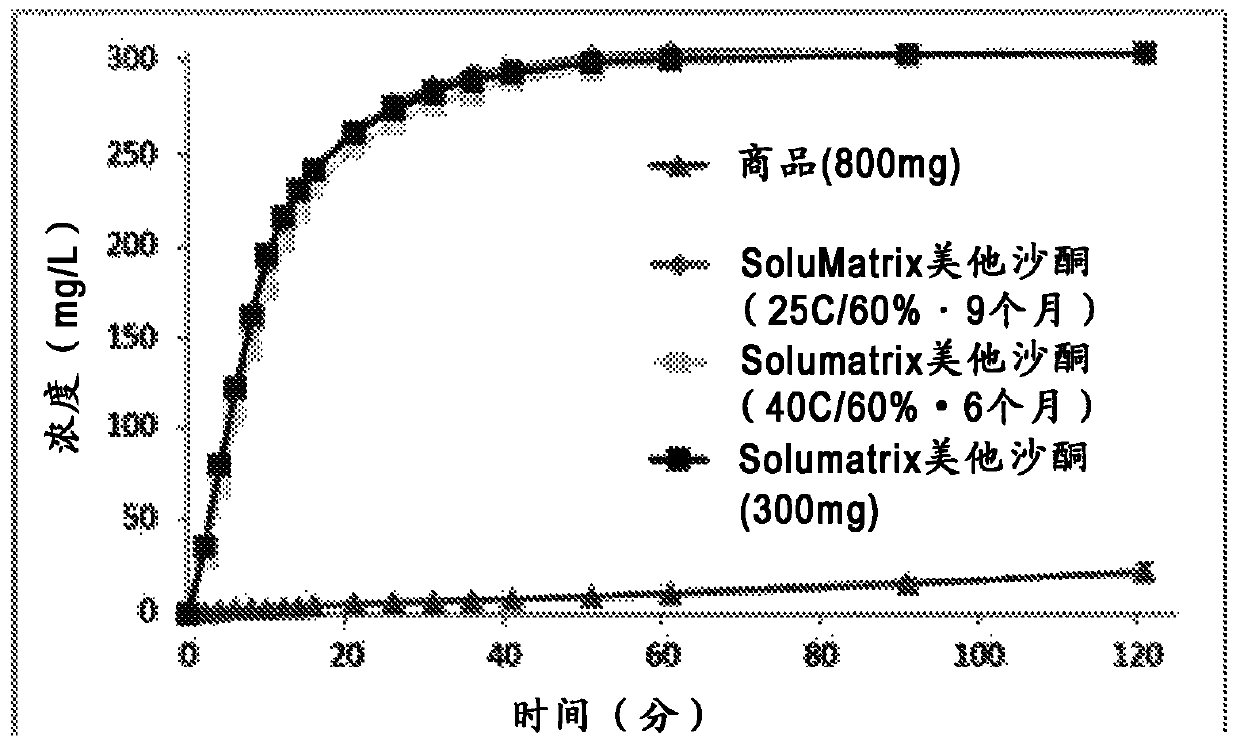
[0117] The milled powder is compressed into tablets using a dry granulation process. Briefly, milled powders were mixed with binders, disintegrants, and lubricants and then transformed into free-flowing granules using a roller compaction system (TFC-Lab Micro, Freund Vector). These granules are mixed with additional disintegrants, binders and lubricants and compressed to produce tablets with a potency of 300 mg. The tablets were tested for dissolution at the initial time point and at 2 and 4 weeks. Stability conditions were 25°C / 60%RH and 40°C / 75%RH. The results of this analysis are described in figure 2 middle. Dissolution was compared to 800 mg Skelaxin tablets. Dissolution was accomplished in a Sotax dissolution apparatus with 1000 ml of 0.01N HCl (pH = 2) at 37°C using a type 2 apparatus (paddle) set at a rotation speed of 100 rpm. An aliquot of the dissolution test solution w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com