Topiroxostat controlled-release granule and preparation method thereof
A technology of topicastat and granules, applied in the field of topicastat controlled-release granules and their preparation, can solve problems such as obvious side effects, and achieve the effects of stable blood drug concentration, reduced irritation, and reduced peak-to-valley phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
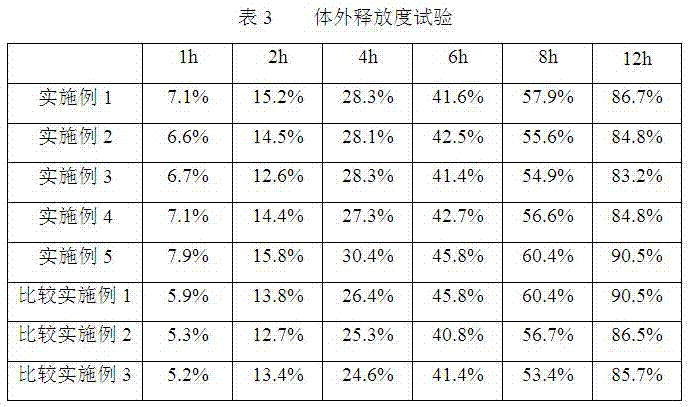
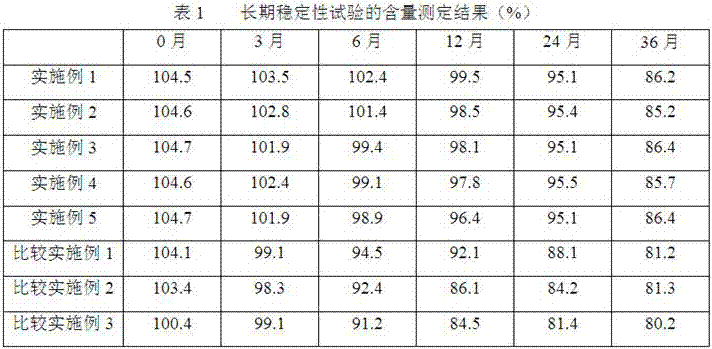
Examples
Embodiment 1
[0040] Topicastat 27.5g
[0041] β-cyclodextrin 106g
[0042] Ethyl cellulose 6.62g
[0043] Sodium carboxymethylcellulose 0.66g
[0044] Su Lisi E-7-1904021.33g
[0045] Hypromellose E50.26g
[0046] Appropriate amount of purified water
[0047] The specific preparation method is as follows:
[0048] (1) Take an appropriate amount of purified water and heat it to 80°C, add β-cyclodextrin and stir to dissolve, then add topinostat, and stir for 30 minutes to form a topinostat solution;
[0049] (2) Regulating spray drier air inlet temperature 160 ℃, air outlet temperature 80 ℃, pressure 0.3MPa, above-mentioned topinostat solution is spray-dried, obtains topinostat soluble powder;
[0050] (3) get 66.25g above-mentioned topinastat soluble powder, ethyl cellulose, sodium carboxymethyl cellulose and mix, add 26.47g water, mix and make soft material;
[0051] (4) Centrifuge-fluidized granulation and coating method to granulate, bake at 60°C for 2 hours, and screen 40-60 mesh...
Embodiment 2
[0054] Topicastat 27.5g
[0055] β-cyclodextrin 106g
[0056] Ethyl cellulose 6.62g
[0057] Sodium carboxymethylcellulose 0.662g
[0058] Su Lisi E-7-1904017.07g
[0059] Hypromellose E50.21g
[0060] Appropriate amount of purified water
[0061] The specific preparation method is as in Example 1.
Embodiment 3
[0063] Topicastat 27.5g
[0064] β-cyclodextrin 96g
[0065] Ethyl cellulose 8.31g
[0066] Sodium carboxymethyl cellulose 0.332g
[0067] Su Lisi E-7-1904024.81g
[0068] Hypromellose E50.49g
[0069] Appropriate amount of purified water
[0070] The specific preparation method is as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com