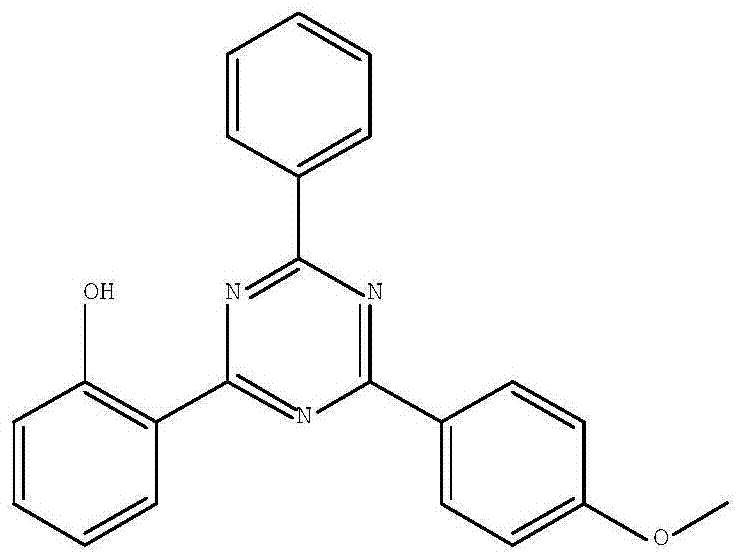
Synthetic method of 2-(2-hydroxyphenyl)-4-(4-methoxyphenyl)-6-phenyl-1,3,5-triazine
A technology of methoxyphenyl and hydroxyphenyl, which is applied in the synthesis field of fine chemicals, can solve problems such as difficulty in dispersing and shortening reaction time, and achieve improved purity and yield, high utilization rate of raw materials, and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
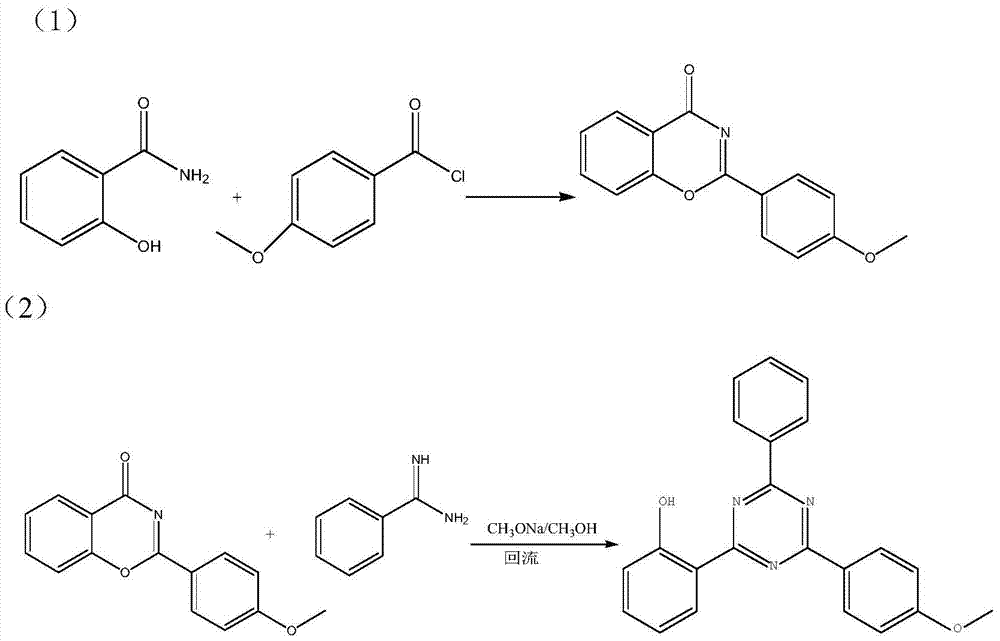
[0033] Example 1: Preparation of intermediate 2-(4-methoxyphenyl)-4 hydrogen-1,3-benzoxazin-4-one
[0034]In a 100mL three-necked flask, add 5g of salicylamide to 13.1g (10.4mL) of 4-methoxybenzoyl chloride at room temperature, and stir at this temperature for 15min (at this time, the reaction solution is a slightly yellow suspension) . Then the temperature is raised to 150-160°C (no strict temperature requirement), and the reaction is kept for one hour (completely dissolved at about 100°C, showing a red clear liquid).
[0035] After the detection reaction is completed, put the material into the flat tray while it is hot. After cooling, it was crushed and used directly in the next reaction to obtain 14.7 g of crude product, which contained 9.2 g of intermediate, and the yield was close to 100%. Elemental Analysis: C 15 h 11 NO 3 : Requires: C71.14%; H4.38%; N5.53%; O18.95%
[0036] Found: C70.94%; H4.43%; N5.42%; O18.99%
Embodiment 2
[0037] Example 2: Preparation of 2-(2-hydroxyphenyl)-4-(4-methoxyphenyl)-6-phenyl-1,3,5-triazine
[0038] Add 750mL of methanol and 20.4g of 30% sodium methoxide into a 1000mL four-necked bottle, stir well, add 5.71g of benzamidine hydrochloride, stir at room temperature for 30min, then add 14.5g at 25°C at one time (slowly add , depending on the exothermic situation) the intermediate in the previous step, after stirring for 30min, the temperature was raised to reflux and kept for 3h. After the TLC detection reaction is completed, cool to 30°C and filter, the filtrate contains methanol / sodium methoxide and can be used directly (apply several times until the impurities increase, the methanol can be evaporated, and the recovery can be applied); the filter cake is washed with water to remove salt, and the salt solution is 30% hydrochloric acid Adjust the pH to 7-8 and stir for 30 minutes (p-methoxybenzoic acid can be recovered); the filter cake is filtered and then washed with me...
Embodiment 3
[0041] Example 3: Preparation of 2-(2-hydroxyphenyl)-4-(4-methoxyphenyl)-6-phenyl-1,3,5-triazine
[0042] Add 375mL of methanol (applicable) and 10.2g of 30% sodium methoxide to a 500mL four-necked bottle, stir well, add 2.86g of benzamidine hydrochloride, stir at room temperature for 30min, then add 7g at 60°C at one time (required for trial production) Slowly add, depending on the exothermic situation) the intermediate in the previous step, stir for 30min, then heat up to reflux and keep for 3h. After the TLC detection reaction is completed, cool to 30°C and filter, the filtrate contains methanol / sodium methoxide and can be used directly (apply several times until the impurities increase, the methanol can be evaporated, and the recovery can be applied); the filter cake is washed with water to remove salt, and the salt solution is 30% hydrochloric acid After adjusting the pH to 7-8, stir for 30 minutes (p-methoxybenzoic acid can be recovered); after the filter cake is filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com