Quick release type medicine phosphatide compound and medicine composition thereof
A phospholipid compound and compound technology, applied in the field of medicine, can solve the problems of decreased liposome stability, decreased drug efficacy, difficulty in obtaining a sufficiently high prototype drug concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
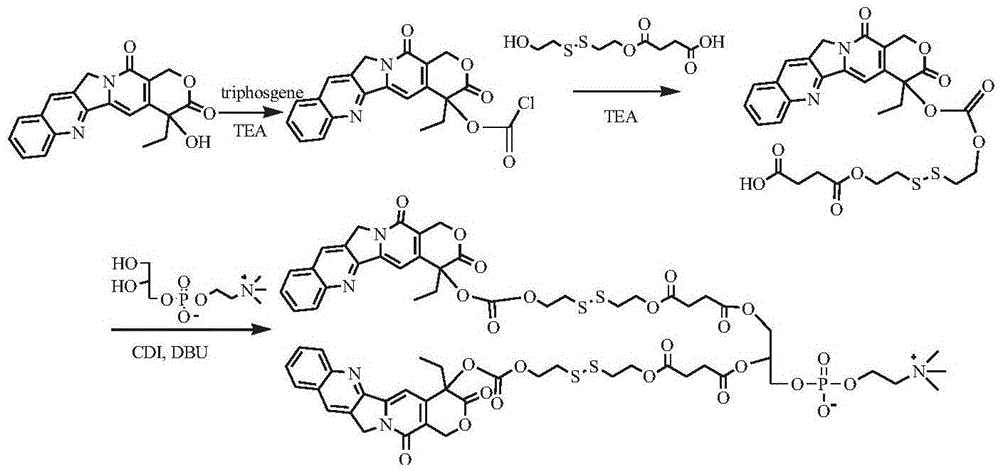
[0141] Synthesis of bis(camptothecin-20-carbonate-dithiodiethylene glycol-succinate) phosphatidylcholine (see the synthetic route figure 1 )
[0142] Dissolve 1 g of camptothecin in 100 mL of chloroform, add 1.0 g of triethylamine, 0.3 g of triphosgene, react at room temperature for 6 h, remove the solvent by rotary evaporation, dissolve the solid in 10 mL of DMSO, add 0.3 g of triethylamine, and add dithio 0.6 g of diethylene glycol succinic acid monoester was reacted at room temperature for 24 hours, and the resulting reaction liquid was purified by column chromatography to obtain 0.65 g of camptothecin-20-carbonate-dithiodiethylene glycol-succinic acid monoester. Dissolve 0.6g of the intermediate camptothecin carbonate-dithiodiethylene glycol-succinic acid monoester in 20mL of DMSO, add 0.6g of CDI, activate for 1h, add 0.3g of GPC and 0.6g of DBU, react at room temperature for 24h, and the obtained reaction The solution was purified by column chromatography to obtain 0.35...
Embodiment 2
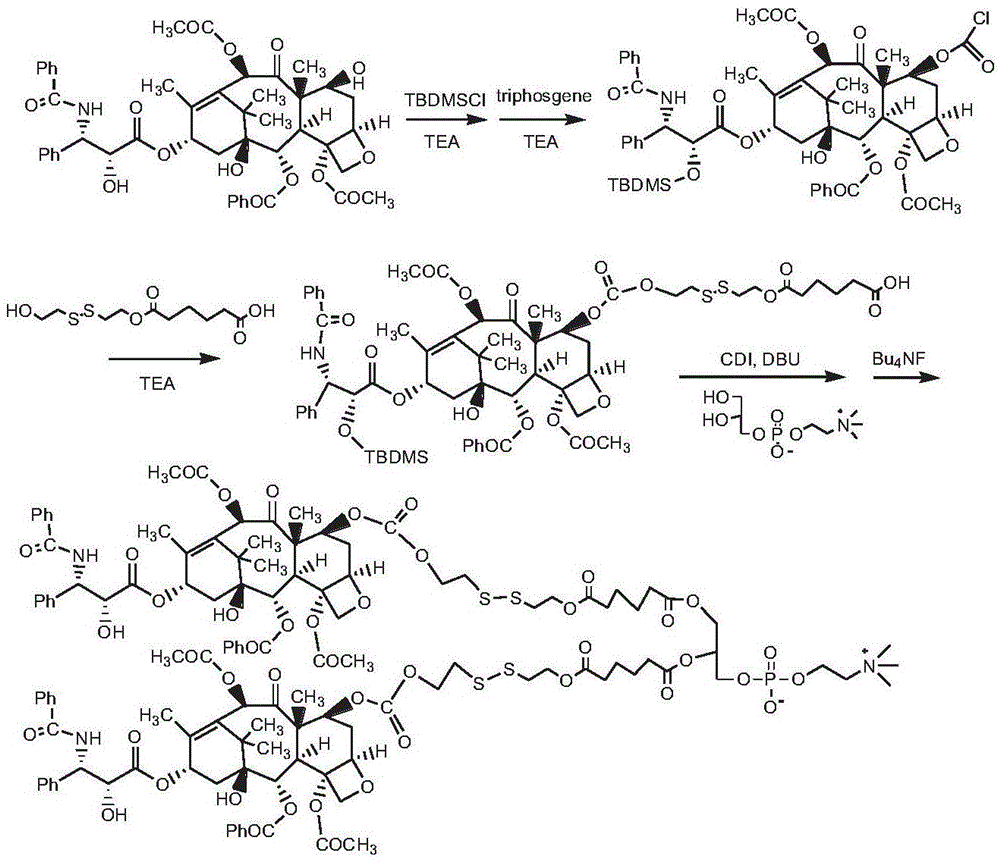
[0146] Synthesis of bis(paclitaxel-7-carbonate-dithiodiethylene glycol-adipate) phosphatidylcholine (route see figure 2 )
[0147] Dissolve 1.0 g of paclitaxel in 30 mL of chloroform, add 0.5 mL of triethylamine, add 0.6 g of tert-butyldimethylsilyl chloride (TBDMSCl), react at 0°C for 3 h, add glacial ether, precipitate a white precipitate, and centrifuge. Further separation by column chromatography (eluent, chloroform / methanol: 7 / 1, v / v) obtained 0.82 g of paclitaxel 2'-blocked product 2'-tert-butyldimethylsilyloxy-paclitaxel, as White powdery solid.
[0148] Dissolve 0.5 g of 2'-tert-butyldimethylsilyloxy-paclitaxel in 50 mL of chloroform, add 0.5 g of triethylamine and 0.2 g of triphosgene, react at room temperature for 6 h, and remove the solvent by rotary evaporation; dissolve the solid in 10 mL of DMSO , add 0.3g of triethylamine, add 0.3g of dithiodiethylene glycol adipate monoester, react at room temperature for 24h, and the resulting reaction solution is purified ...
Embodiment 3
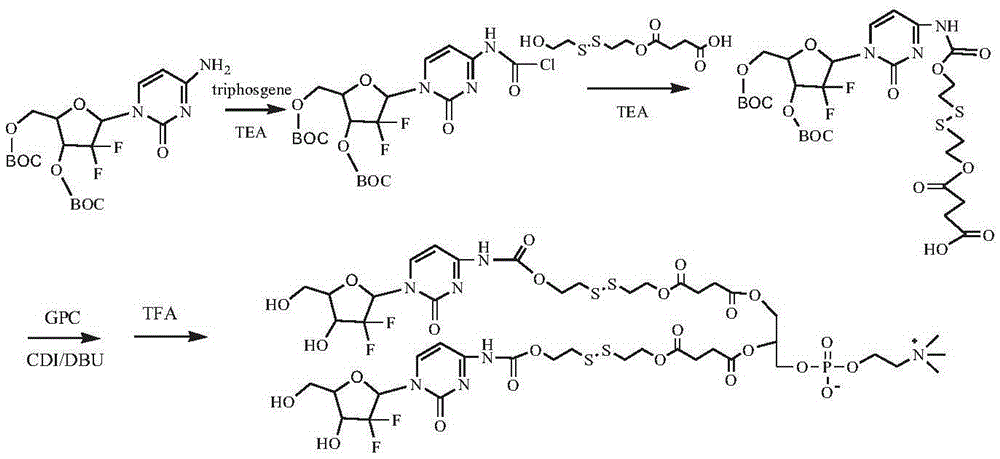
[0152] Synthesis of bis(gemcitabine-4-N-carbamate-dithiodiethylene glycol-succinate) phosphatidylcholine (see the synthetic route image 3 )
[0153] The gemcitabine derivative 3'-O-BOC-5'-O-BOC-gemcitabine protected by tert-butoxycarbonyl (BOC) was synthesized according to the method in the literature (J.Org.Chem.1999, 64, 8319-8322).
[0154] Dissolve 1 g of 3′-O-BOC-5′-O-BOC-gemcitabine in 100 mL of chloroform, add 0.6 g of triethylamine and 0.3 g of triphosgene, react at room temperature for 6 h, and remove the solvent by rotary evaporation; dissolve the solid in 10 mL of DMSO , add 0.3 g of triethylamine, add 0.6 g of dithiodiethylene glycol succinic acid monoester, react at room temperature for 24 hours, and purify the resulting reaction solution by column chromatography to obtain 3'-O-BOC-5'-O-BOC - Gemcitabine-4-N-carbamate-dithiodiethylene glycol-succinic acid monoester 0.72g; intermediate 3'-O-BOC-5'-O-BOC-gemcitabine-4-N-amino Dissolve 0.6g of formate-dithiodiethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com