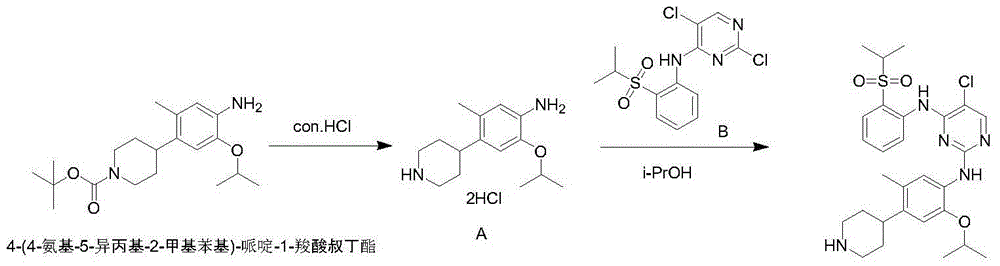
New synthesis technology of ceritinib intermediate
A technology for ceritinib and intermediates, which is applied in the field of new preparation of ceritinib intermediate 4--piperidine-1-carboxylate tert-butyl ester, which can solve the problem of increased manufacturing costs, high dosage, and difficulty in industrialization and other problems, to achieve the effect of short steps, low cost of raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
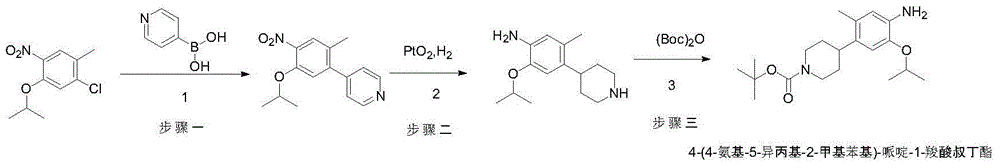
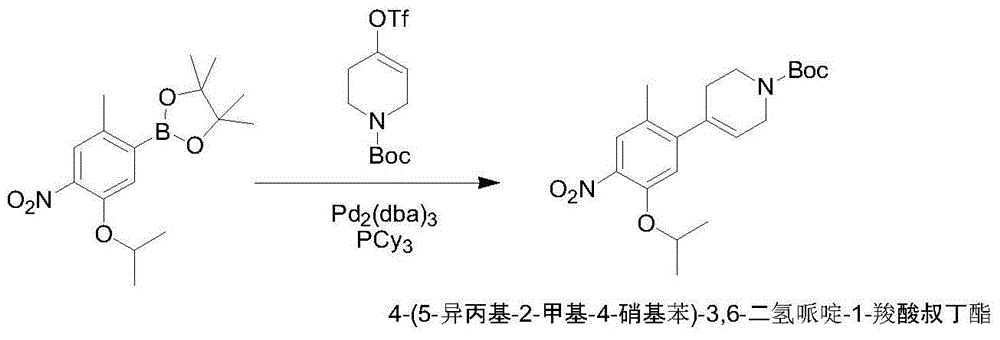
[0020] Preparation of tert-butyl 4-(5-isopropyl-2-methyl-4-nitrobenzene)-3,6-dihydropiperidine-1-carboxylate
[0021] 1-Chloro-5-isopropyl-2-methyl-4-nitrobenzene (25.3g, 0.11mol), N-tert-butoxy-1,2,5,6-tetrahydropyridine-4-boronic acid Pinacol ester (30.9g, 0.1mol), potassium carbonate (13.8g, 0.1mol), tetrakistriphenylphosphine palladium (1.15g, 0.001mol, 1% eq.) were added to toluene / water (220mL / 220mL) in sequence In the reaction mixture, the reaction mixture was stirred at 70-80 degrees Celsius for 5 hours (under nitrogen protection), TLC showed that the reaction was complete, the reaction mixture was cooled to 20-30 degrees Celsius, suction filtered, and n-heptane (220 mL) was added to the filtrate , layered, after the organic layer was concentrated to dry solvent, n-heptane (220mL) was added to the residue, stirred at 0-10 degrees Celsius for 2 hours, filtered with suction, and the filter cake was vacuum-dried at 40-50 degrees Celsius to obtain off-white powder tert-bu...
Embodiment 2
[0023] Preparation of tert-butyl 4-(5-isopropyl-2-methyl-4-nitrobenzene)-3,6-dihydropiperidine-1-carboxylate
[0024] 1-Chloro-5-isopropyl-2-methyl-4-nitrobenzene (25.3g, 0.11mol), N-tert-butoxy-1,2,5,6-tetrahydropyridine-4-boronic acid Pinacol ester (30.9g, 0.1mol), potassium phosphate (21.2g, 0.33mol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (58.48g, 0.08mol , 0.8eq.) were sequentially added to the 1,4-dioxane / water (220mL / 220mL) reaction mixture, the reaction mixture was stirred at 90-101 degrees Celsius for 4 hours (under nitrogen protection), TLC showed that the reaction was complete, and the reaction mixture Cool the liquid to 20-30 degrees Celsius, filter with suction, add n-heptane (220mL) to the filtrate, and separate the layers. After the organic layer is concentrated to dryness, add n-heptane (220mL) to the residue, Stir for 2 hours, filter with suction, and vacuum-dry the filter cake at 40-50 degrees Celsius to obtain off-white powder 4-(5-isopr...
Embodiment 3
[0026] Preparation of tert-butyl 4-(5-isopropyl-2-methyl-4-nitrobenzene)-3,6-dihydropiperidine-1-carboxylate
[0027] 1-Chloro-5-isopropyl-2-methyl-4-nitrobenzene (25.3g, 0.11mol), N-tert-butoxy-1,2,5,6-tetrahydropyridine-4-boronic acid Pinacol ester (30.9g, 0.1mol), potassium carbonate (13.8g, 0.10mol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (0.22g, 0.0003mol , 0.3% eq.) was added to N,N-dimethylformamide / water (220mL / 220mL) reaction mixture in sequence, and the reaction mixture was stirred at 90-101 degrees Celsius for 4 hours (under nitrogen protection), and TLC showed that the reaction was complete , the reaction mixture was cooled to 20-30 degrees Celsius, suction filtered, and n-heptane (220mL) was added to the filtrate, and the layers were separated. After the organic layer was concentrated to dry solvent, n-heptane (220mL) was added to the residue, Stir at -10°C for 2 hours, filter with suction, and dry the filter cake in vacuum at 40-50°C to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com