Oxdiazole compound solvate and preparation method thereof
A solvate and oxadiazole technology, applied in the field of drug crystals, can solve the problems of unfavorable crystal form stability, storage stability of preparations, affecting the operability of preparations, difficulty in filtration and drying, etc., to reduce the risk of curative effect decline and safety risks , Improve bioavailability, fast dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0075] Preparation Example 1 (preparation of known ataluren)
[0076] Ataluren can be prepared according to the method described in Example 2 of patent document WO2004091502A2.
[0077] The specific preparation method is:
[0078] Add 62.19 g of potassium carbonate to 44.14 g of 3-cyanobenzoic acid dissolved in 0.6 liter of DMF, and stir at room temperature for 30 minutes. To the suspension was added 28 ml (450 mmol) of iodomethane over 20 minutes, and the reaction mixture was stirred at room temperature for 4 hours. The reaction mixture was poured into 1.2 liters of ice water, stirred for 30 minutes, and the precipitate was filtered off. The white filter cake was dissolved in 70 mL of methanol and reprecipitated in cold water. Methyl 3-cyanobenzoate was obtained in a yield of 79%.
[0079] 50 g of methyl 3-cyanobenzoate was dissolved in 500 ml of ethanol, and 41 ml of 50% aqueous hydroxylamine (620 mmol) was added thereto. The reaction mixture was stirred at 100°C for ...
preparation example 2
[0083] Preparation example 2 (preparation of known A crystal form)
[0084] The known crystal form A can be prepared according to the method described in Example 5.1.1.1 of patent document WO2008039431A2. Specifically: add 100 mg of ataluren prepared in Preparation Example 1, add 16.2 ml of isopropanol to sonicate at 60 ° C, filter the solution through a 0.2 micron filter, place the filtrate in a vial covered with aluminum foil with small holes, and 60 °C evaporated. The solid formed was isolated to give crystalline form A of ataluren.
[0085] It is acicular crystals.
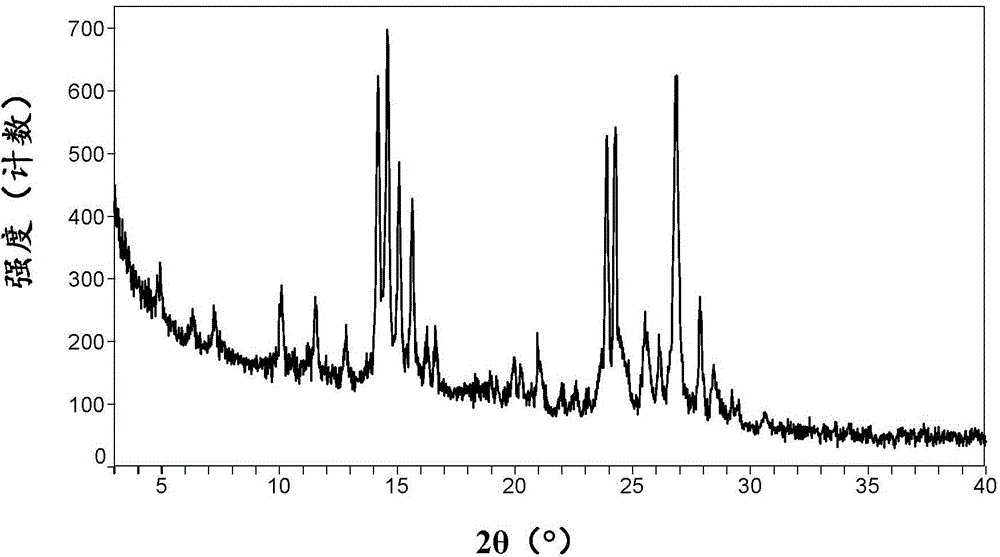
[0086] Its XRPD pattern is shown in figure 1 , showing that it is consistent with the A crystal form of ataluren disclosed in the patent document WO2008039431A2.
preparation example 3
[0087] Preparation example 3 (preparation of known crystal form B)
[0088] The known crystal form B can be prepared according to the method described in Example 5.1.2.1 of patent document WO2008039431A2. Specifically: add 50 mg of ataluren prepared in Preparation Example 1, add 20 ml of acetone at 25 ° C to dissolve it ultrasonically, filter the solution through a 0.2 micron filter, place the filtrate in a vial covered with aluminum foil with small holes, and evaporate at 50 ° C . The solid formed was isolated to give crystalline form B of ataluren.
[0089] It is acicular crystals.
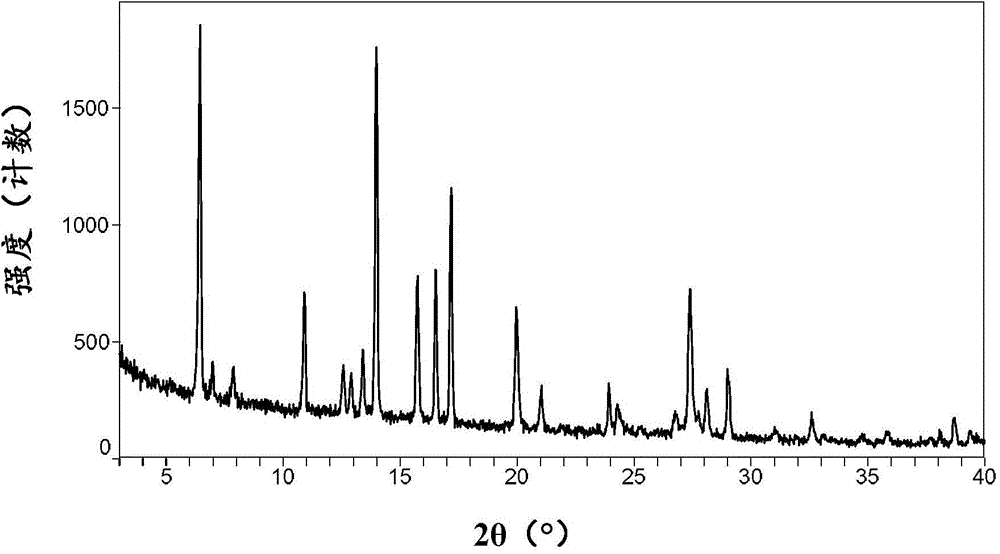
[0090] Its XRPD pattern is shown in figure 2 , showing that it is consistent with the B crystal form of ataluren disclosed in the patent document WO2008039431A2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com