Pressure-sensitive adhesive composition
A technology of pressure-sensitive adhesives and pressure-sensitive adhesive layers, applied in the direction of adhesive types, ester copolymer adhesives, adhesives, etc., which can solve the problems of shortened pot life and process stability of pressure-sensitive adhesives , Decreased storage stability and other issues, to achieve the effect of shortening the curing time, excellent antistatic property, and excellent antistatic property
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0082] Synthesis example 1. Synthesis of 5-hydroxypentyl acrylate
[0083] 【Chemical 3】
[0084] [Reaction 1]
[0085]
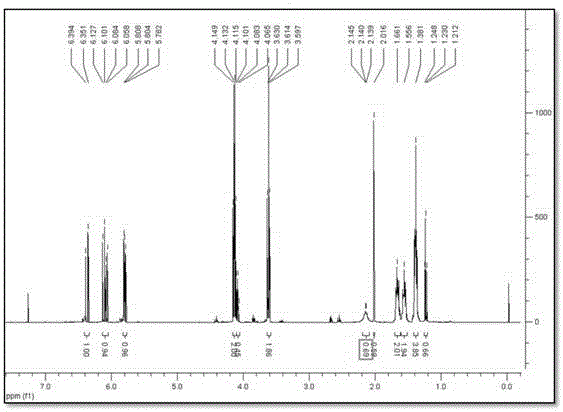
[0086] Dissolve 1,5-dihydroxypentane (10.5 g, 0.1 mol) in pyridine (100 mL), drop acryloyl chloride (9.05 g) from the dropping funnel over 10 minutes, stir at room temperature for 1 hour, and add Distilled water (200mL) and ethyl acetate (200mL) were extracted into the organic layer, and after vacuum distillation of the extracted organic layer, impurities and dimers were separated and removed by column chromatography, and the solvent was distilled off under reduced pressure to prepare acrylic acid 5 -Hydroxypentyl ester (8.7 g, yield 46.3%).
[0087] Dissolve the prepared compound in CDCl 3 Solvent, confirm NMR, confirm to be the 5-hydroxypentyl acrylate of purity more than 98% ( figure 1 ).
Synthetic example 2
[0088] Synthesis example 2. Synthesis of 6-hydroxyhexyl acrylate
[0089] 【Chemical 4】
[0090] [Reaction 2]
[0091]
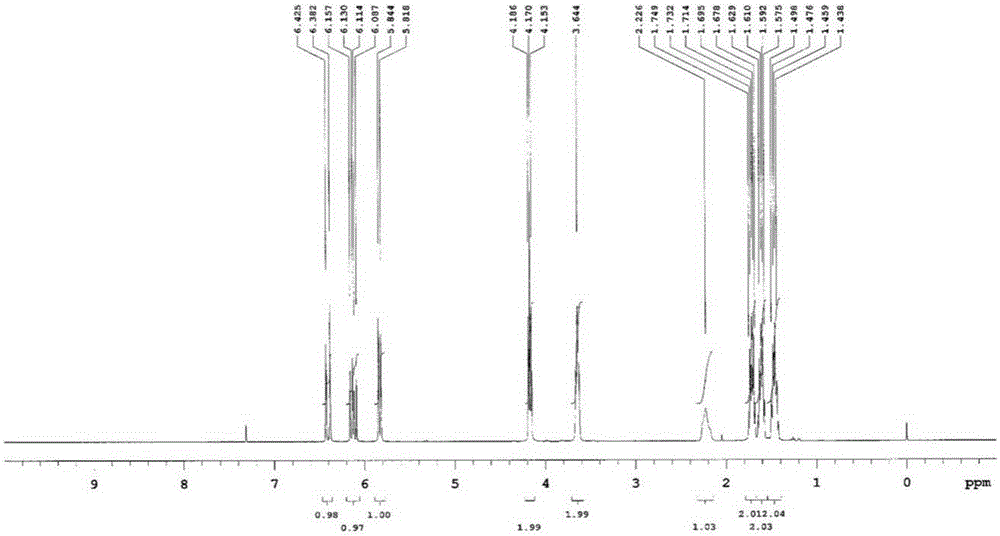
[0092] Dissolve 1,6-dihydroxyhexane (11.8 g, 0.1 mol) in pyridine (100 mL), add acryloyl chloride (9.05 g) dropwise from the dropping funnel over 10 minutes, stir at room temperature for 1 hour, and then add distilled water (200mL) and ethyl acetate (200mL), extracted with an organic layer, and after vacuum distillation of the extracted organic layer, use column chromatography to separate and remove impurities and dimers to prepare 6-hydroxyhexyl acrylate (10.5g, Yield 61.0%).
[0093] Dissolve the prepared compound in CDCl 3 Solvent, confirm NMR, confirm to be the 6-hydroxyhexyl acrylate of purity more than 98% ( figure 2 ).
manufacture example 1
[0094] Production example 1. Production of acrylic copolymer A-1
[0095] In a 1 L reactor with nitrogen reflux and a cooling device installed to facilitate temperature regulation, 98 parts by weight of n-butyl acrylate (BA) and 2 parts by weight of 5-hydroxypentyl acrylate (Synthesis Example 1) were charged. 100 parts by weight of ethyl acetate (EA) was added as a solvent after the monomer mixture composed of 2.5 parts. Then, in order to remove oxygen, nitrogen gas was introduced for 1 hour to replace, and the temperature was maintained at 70°C. After uniformly stirring the monomer mixture, 0.07 parts by weight of azobisisobutyronitrile (AIBN) was added as a reaction initiator, reacted for 8 hours, and ethyl acetate was further added to produce an acrylic copolymer A-1.
[0096] It was confirmed that the obtained acrylic copolymer had a solid content of 40%, a weight average molecular weight of 800,000, a PDI of 7.6, and a viscosity of 11,500 cP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com