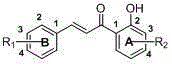
Carbamate chalcone compound, preparation method therefor and use of carbamate chalcone compound
A technology of chalcone ester and carbamic acid, which is applied in the field of medicinal chemistry of the Ming Dynasty, and can solve problems such as unsatisfactory depolymerization activity and poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
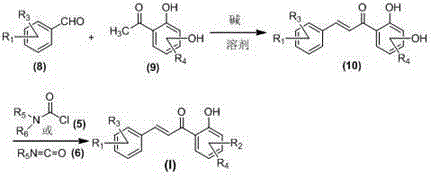
[0047] The preparation general method of embodiment 1 carbamic acid chalcone ester compound (I)
[0048] Add 2.0mmol corresponding 2-hydroxyacetophenone compound ( 2 ), 3.0mmol corresponding benzaldehyde compounds ( 1 ), 8.0mmol of anhydrous potassium carbonate and 50ml of acetonitrile, after stirring evenly, the temperature was raised to reflux and stirred for 2.0 to 72.0 hours (the reaction process was tracked by TLC); after the reaction was completed, it was cooled to room temperature, and the pH of the reaction solution was adjusted with 10% hydrochloric acid aqueous solution to Strong acidity, then use saturated aqueous sodium bicarbonate solution to adjust the pH of the reaction solution to weak alkalinity, evaporate acetonitrile under reduced pressure, add 80mL deionized water to the residual liquid, extract three times with 300mL dichloromethane, combine the organic layers with saturated chlorine Wash with aqueous sodium chloride solution, dry over anhydrous sodium ...
Embodiment 2
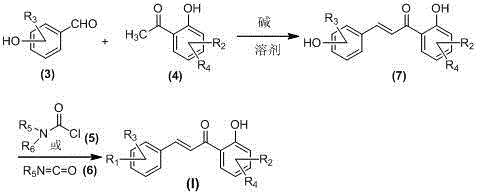
[0058] General method for the preparation of embodiment 2 carbamic acid chalcone ester compound (I)
[0059] Add 2.0mmol corresponding 2-hydroxyacetophenone compound ( 4 ) or dihydroxyacetophenones ( 9 ), 3.0mmol corresponding hydroxybenzaldehyde compounds ( 3 ) or benzaldehyde compounds ( 8 ), and 40ml ethanol, after stirring evenly, add 8.0mmol of 30% KOH aqueous solution dropwise, and stir at room temperature for 2.0-72.0 hours (reaction progress is tracked by TLC); after the reaction, adjust the pH of the reaction solution to strong acidity with 10% hydrochloric acid aqueous solution , then use saturated aqueous sodium bicarbonate solution to adjust the pH of the reaction solution to weak alkalinity, evaporate ethanol under reduced pressure, add 80mL deionized water to the residual liquid, extract three times with 300mL dichloromethane, combine the organic layers with saturated sodium chloride Wash with aqueous solution, dry over anhydrous sodium sulfate, filter, evap...
Embodiment 3
[0068] Example 3 Chalcone Carbamic Acid Esters (I) and Acid Salt Preparation General Method
[0069] In the reaction flask, add the chalcone carbamate compound ( I ) 2.0mmol and 50ml of acetone, after stirring evenly, add 8.0mmol of the corresponding acid, heat up and reflux and stir for 20 minutes, cool to room temperature after the reaction, evaporate the solvent under reduced pressure, recrystallize the residue with acetone, filter the precipitated solid, that is Chalcone carbamate compounds ( I ) of the salt, its chemical structure by 1 Confirmed by HNMR and ESI-MS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com