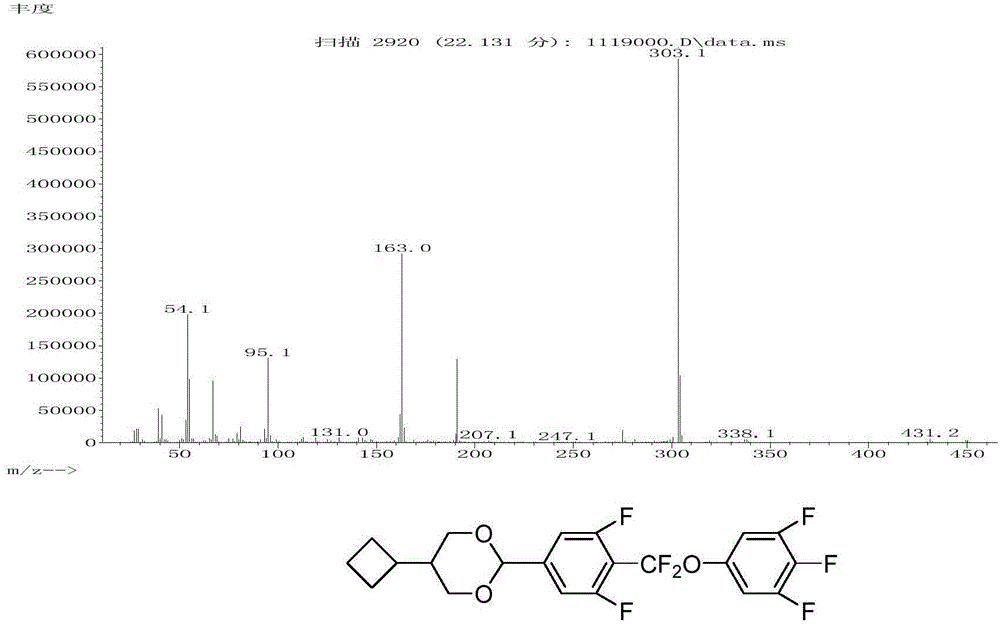
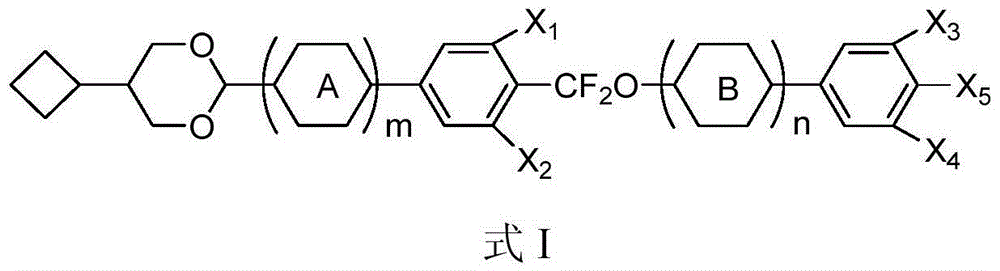
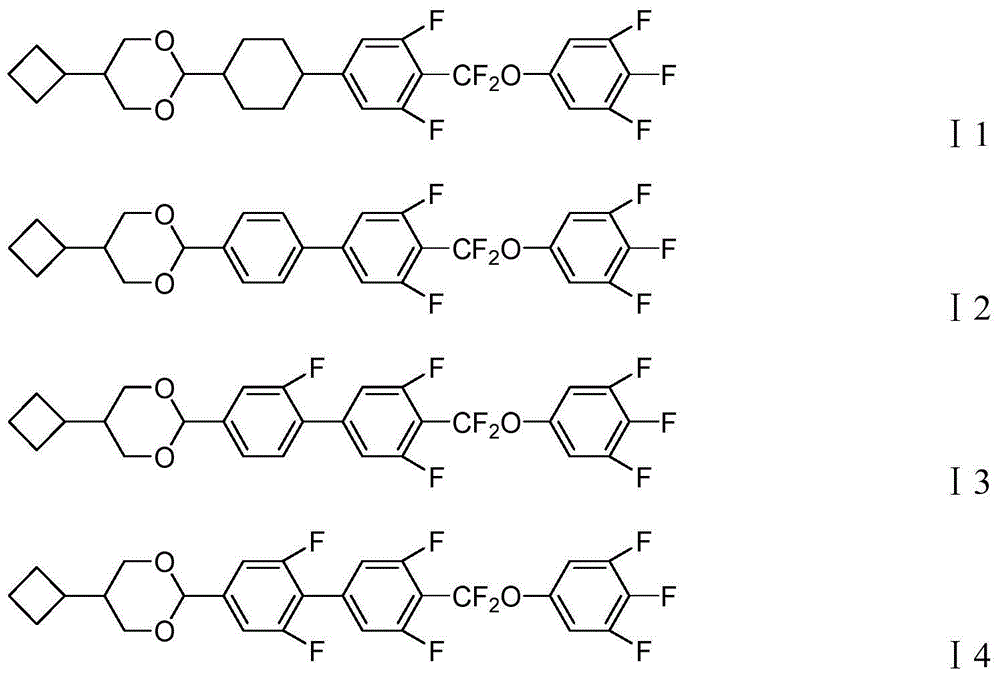
Liquid crystal compound containing 5-cyclobutyl-1,3-dioxy cyclohexane structure, preparation method and applications thereof
A compound and liquid crystal technology, applied in chemical instruments and methods, organic chemistry, liquid crystal materials, etc., can solve the problems of lowering the clearing point of liquid crystals, restricting the space for increasing the response speed of liquid crystal mixtures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The general preparation method of the compound represented by formula I provided by the present invention is shown in Synthetic Scheme 1.
[0089] Wherein, the preparation method of the compound represented by formula II is shown in the synthetic route 2,3.
[0090] The principle, operation process, conventional post-treatment, silica gel column, recrystallization and purification of such methods are well known to those skilled in the art. According to the following introduction, the synthesis process can be completely realized and the target product can be obtained.
[0091] The reaction process generally monitors the progress of the reaction by TLC. The post-treatment after the reaction is generally washed with water, extracted, combined with organic phases, dried, evaporated under reduced pressure to remove solvent, and recrystallization, column chromatography. Those skilled in the art can follow The following description implements the present invention.
[0092] R...
Embodiment 1
[0124] Embodiment 1, compound shown in preparation formula I1 (route 3)
[0125]
[0126] step 1
[0127]
[0128] Add 17.7g (0.77mol) sodium silk in batches to 450ml of absolute ethanol, stir until the sodium completely disappears, heat to reflux, add 112g (0.7mol) diethyl malonate dropwise, reflux for 1 hour and cool to 60°C, drop Add 104g (0.77mol) cyclobutyl bromide, reflux for 4 hours after dripping, pour into an equivalent amount of sherwood oil and water, stir and let stand, separate liquids, wash with water, dry under reduced pressure over anhydrous sodium sulfate, and collect the compound (1 -a) 105g, yield 70%.
[0129] step 2
[0130]
[0131] 40.5g (0.75mol) of potassium borohydride and 31.9g (0.75mol) of anhydrous lithium chloride were added to 500ml of tetrahydrofuran, heated to reflux under nitrogen protection, heating was stopped, and 53.5g (0.25mol) of compound (1-a) was added dropwise. After dropping, heat and reflux for 12 hours, pour the reaction ...
Embodiment 2
[0159] Embodiment 2, compound shown in preparation formula I2 (route 2)
[0160]
[0161] step 1
[0162]
[0163] Using p-bromobenzaldehyde and ethylene glycol as raw materials, refer to Step 9 of Example 1 to obtain product (2-a).
[0164] step 2
[0165]
[0166] Dissolve (2-a) 50.8g (0.222mol) and 39g (0.244mol) of 3,5-difluorophenylboronic acid obtained in step 1 in a mixed solution of 600ml of toluene, 300ml of ethanol and 300ml of water, and add 27g (0.256mol) Sodium carbonate, heated and stirred under nitrogen protection, added 2g tetrakistriphenylphosphine palladium during reflux, stopped the reaction in 7 hours, separated liquids, extracted the water layer with toluene, combined the toluene layers, spin-dried the solution, petroleum ether column chromatography, spin-dried After drying the solvent, it was recrystallized with petroleum ether to obtain 50 g of product (2-b) with a yield of 86%.
[0167] Step 3 is obtained with reference to step 5 of Example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com