L-arabinose isomerase and application thereof in production of L-ribulose
An arabinose and isomerase technology, applied in the field of bioengineering, can solve the problem of low catalytic efficiency of L-arabinose, and achieve wide application prospects and the effect of economic value and high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Extraction of Paenibacillus polymyxa NX-1 genomic DNA.
[0052] Genomic DNA of Bacillus polymyxa NX-1 in logarithmic growth phase was extracted with GenomicDNA Purification Kit (Takara, Dalian), and the obtained bacterial genome was detected by agarose gel electrophoresis.
Embodiment 2
[0053] Example 2: Cloning of the gene encoding L-arabinose isomerase (araA) and construction of recombinant bacteria.
[0054] 2.1 PCR amplification of araA gene
[0055] According to the sequence of Bacillus polymyxa L-AI gene reported on GeneBank, primers Primer1 and Primer2 were designed using VectorNTI software. The primer sequence is:
[0056] Primer 1: 5'-CG GGATCC (BamHI) ATGTCAACAGTAAGTACAAAACAGT-3';
[0057] Primer 2: 5'-ATAAGAAT GCGGCCGC (NotI) TTATTTAATTATTACGTATTCCAGG-3';
[0058] Using the genomic DNA of Bacillus polymyxa obtained in Example 1 as a template, the gene fragment of Bacillus polymyxa was amplified.
[0059] The PCR amplification system (25 μL) is: 2 μL of genomic DNA, 1 μL of each primer 1 and primer 2, 2 μL of dNTP, 2.5 μL of 10×Tag buffer, 0.5 μL of Ex-Tag polymerase, ddH 2 O 16 μL;
[0060] The PCR reaction program was as follows: step 1: pre-denaturation at 94°C for 2 minutes; step 2: denaturation at 94°C for 2 minutes; then annealing at 55...
Embodiment 3
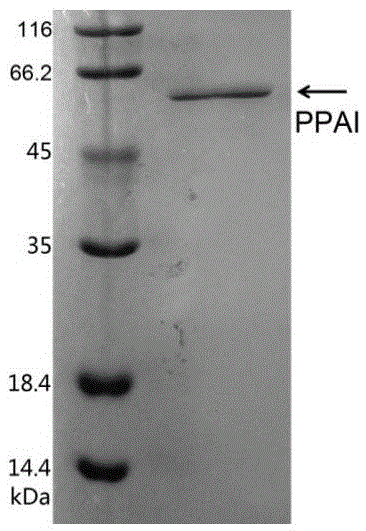
[0077] Example 3: Induced expression of L-arabinose isomerase.
[0078] Inoculate the recombinant Escherichia coli BL21(DE3)-AI in 5 mL of LB liquid medium supplemented with 25 μg / mL kanamycin, cultivate overnight at 37°C on a shaker; Put it into a 500mL shake flask filled with 100mL LB medium (containing 25μg / mL kanamycin), and culture it on a shaking table at 37°C for 2-3h, until the OD 600 Add IPTG at about 0.6-1.0 for induction (IPTG final concentration 1mM), or add 1g / L lactose for induction, and then continue to induce expression for 6h, and collect the bacteria by centrifugation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com