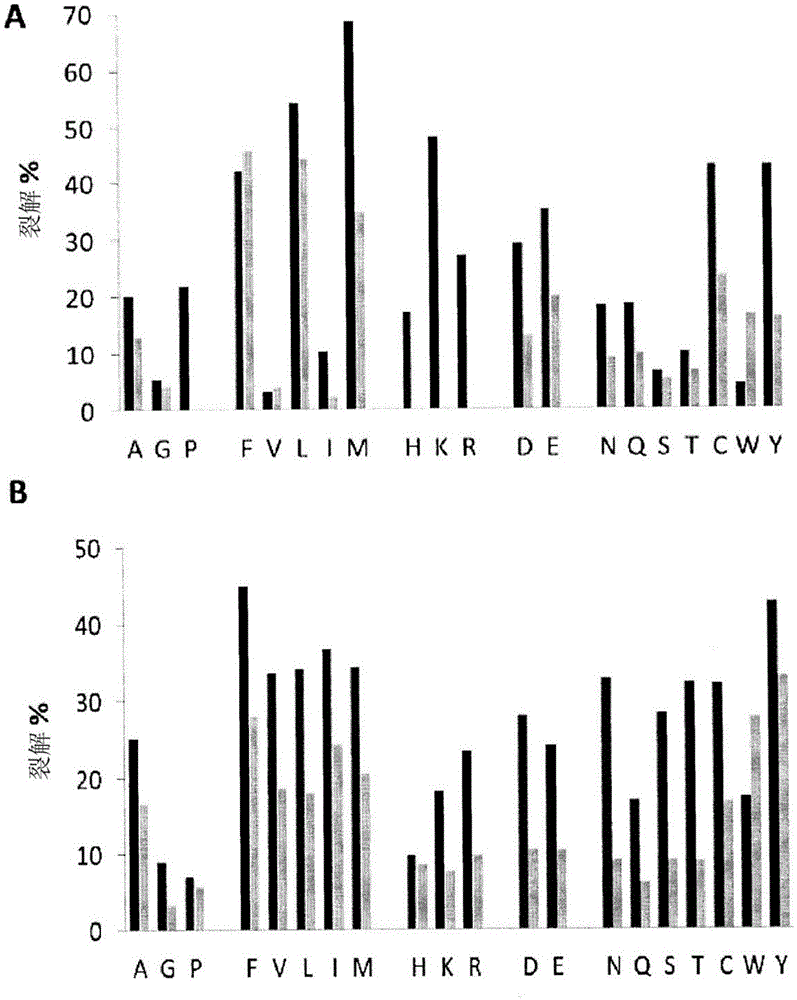Treatment of gluten intolerance and related conditions
A technology for gluten intolerance and related diseases, which is applied in the field of compositions for treating gluten intolerance and related diseases, and can solve problems such as bacteria species that are difficult to eradicate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Preparation of Nepenthes Protease Extract
[0158] Chemicals
[0159] HPLC grade water and acetonitrile from Burdick and Jackson were purchased from VWR. Formic acid, Tris, glycine were purchased from SigmaAldrich.
[0160] plant culture
[0161] Transplants of Nepenthes rafflesiana, Nepenthes ampularia, Nepenthes mirabilis and Nepenthes globosa were purchased from Keehns Carnivores (http: / / www.keehnscarnivores.ca) . These transplanted plants were cultivated with veneer, perlite, peat moss and humus (40%, 35%, 10%, 5%, respectively). The growing conditions include 14 hours of light per day, 80% humidity and a temperature of 23°C to 28°C and watering two to three times a week. After the pitcher leaves of Nepenthes matured, the plants were fed with one or two flies per pitcher, and pitcher sap was harvested one week later. The recovery of pitcher leaves and their secretions lasted one week, followed by a second round of feeding and extraction.
[0162] Extract prep...
Embodiment 2
[0167] Protein Digestion Mapping of Pepsin and Nepenthes Protease Extracts
[0168] Digestion mapping of nepenthes proteases by mass spectrometry
[0169] Nepenthes protease extract was prepared as in Example 1.
[0170] Protein digestion was performed in solution using a LEAPHTX-PAL autosampler and a distribution system designed for hydrogen / deuterium exchange (HDX) applications, and data was collected using an ABSciex Triple-TOF5600QqTOF mass spectrometer. Peptides were identified by MS / MS data using Mascot (v2.3). Briefly, 8 μL, 8 μM protein (XRCC4, XLF, Ligase IV-tandem BRCT domain, PNK, myoglobin or cytochrome C) was mixed with 10 μL, 11-fold concentrated liquid for 2 min at 10°C. Myoglobin and cytochrome C were purchased from Sigma. After dilution to 1 μM substrate concentration, 15 μL was injected into a refrigerated reverse phase LC system (4° C.) connected to a mass spectrometer. Peptides were captured on a 5 cm, 200 μm.d. Onyx C18 monolithic column (Phenomenex In...
Embodiment 3
[0175] Digestion mapping of the multidomain protein XRCC4 by pepsin and nepenthes protease extracts
[0176] HD exchange of complexes involved in DNA damage repair
[0177] Nepenthes protease extract was prepared as in Example 1.
[0178] Stock solutions of XRCC4 (1-200) containing BRCT as well as XRCC4 (full length) containing BRCT were diluted to equimolar concentration (10 μM) in buffer (10 mM Tris-HCl, pH 7.5) and incubated at 4°C for minimal 30 minutes for enhanced complexation. Samples were kept at 4°C until HDX analysis. Aliquot the sample at 20 °C by adding D 2O (25% v / v) deuterated for 2 minutes. Aliquots were subsequently digested in two ways. In the first digestion strategy, protein deuteration was quenched by adding the sample to chilled 100 mM glycine-HCl, pH 2.5, and the quenched protein solution was injected into a pepsin microreactor. The microreactor was installed in the HTX-PAL system between the injector valve and the C18 column. Protein digests were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com