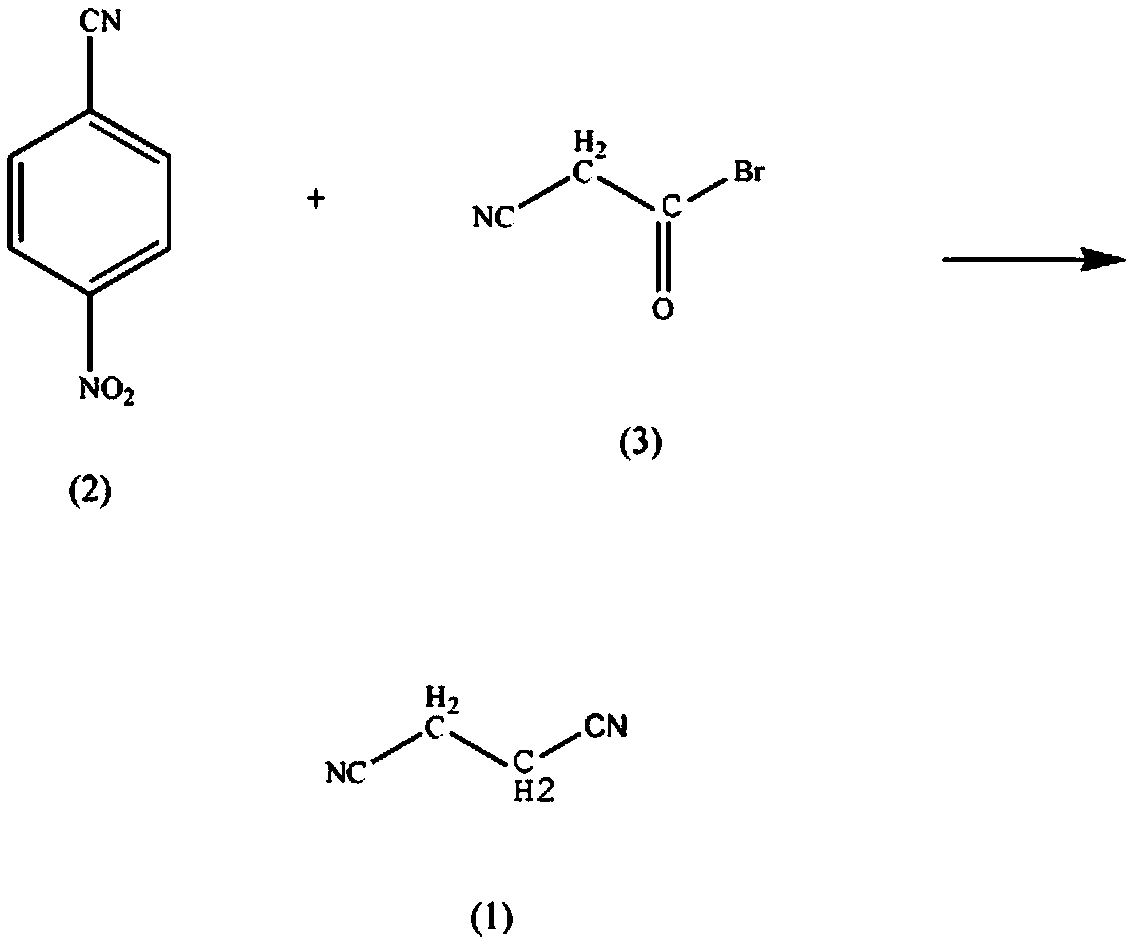
Synthetic method for methotrexate drug intermediate malononitrile
A technology of malononitrile and methotrexate, which is applied in the field of synthesis of methotrexate drug intermediate malononitrile, can solve the problem of weak cell action in G1 phase, reduce reaction temperature and reaction time, and reduce intermediate link, the effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] In the reaction vessel that reactor, thermometer, dropping funnel are installed, add cyanoacetyl bromide (2) 3.9mol, cuprous chloride 0.56mol, mass fraction is 70% 4-nitro-benzonitrile solution ( 3) 4.6-4.9mol, mass fraction is 50% cyclohexane 500ml, control the stirring speed at 150rpm, increase the solution temperature to 50°C, react for 5h, keep the pressure at 8kPa, raise the solution temperature to 80°C, react for 90min, liter Raise the solution temperature to 130°C, react for 4-6h, lower the solution temperature to 10°C, precipitate solids, filter, wash with 40% acetonitrile by mass fraction, wash with 40% ethylenediamine by mass fraction, and dehydrate activated alumina. Recrystallized from 90% nitromethane to obtain 185.33 g of crystalline malononitrile with a yield of 72%.
example 2
[0014] In the reaction vessel that reactor, thermometer, dropping funnel are installed, add cyanoacetyl bromide (2) 3.9mol, cuprous chloride 0.56mol, massfraction is 72% 4-nitro-benzonitrile solution ( 3) 4.7mol, 500ml of cyclohexane with a mass fraction of 52%, control the stirring speed at 170rpm, raise the solution temperature to 52°C, react for 7h, keep the pressure at 9kPa, raise the solution temperature to 82°C, react for 110min, raise the solution Temperature to 132°C, react for 5 hours, lower the solution temperature to 12°C, precipitate solid, filter, wash with acetonitrile with a mass fraction of 425%, wash with ethylenediamine with a mass fraction of 42%, and dehydrate the solid sodium hydroxide at a mass fraction of 92% Recrystallized in nitromethane to obtain 203.35 g of crystalline malononitrile with a yield of 79%.
example 3
[0016] In the reaction vessel that reactor, thermometer, dropping funnel are installed, add cyanoacetyl bromide (2) 3.9mol, cuprous chloride 0.56mol, mass fraction is 75% 4-nitro-benzonitrile solution ( 3) 4.9mol, 500ml of cyclohexane with a mass fraction of 55%, control the stirring speed at 190rpm, raise the solution temperature to 55°C, react for 8h, keep the pressure at 10kPa, raise the solution temperature to 85°C, react for 130min, raise the solution Temperature to 135°C, react for 6h, lower the solution temperature to 15°C, precipitate solid, filter, wash with 45% acetonitrile by mass fraction, wash with 45% ethylenediamine by mass fraction, dehydrate activated alumina, and dehydrate at 95% nitric acid Recrystallized from methyl methane to obtain 211.07 g of crystalline malononitrile with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com