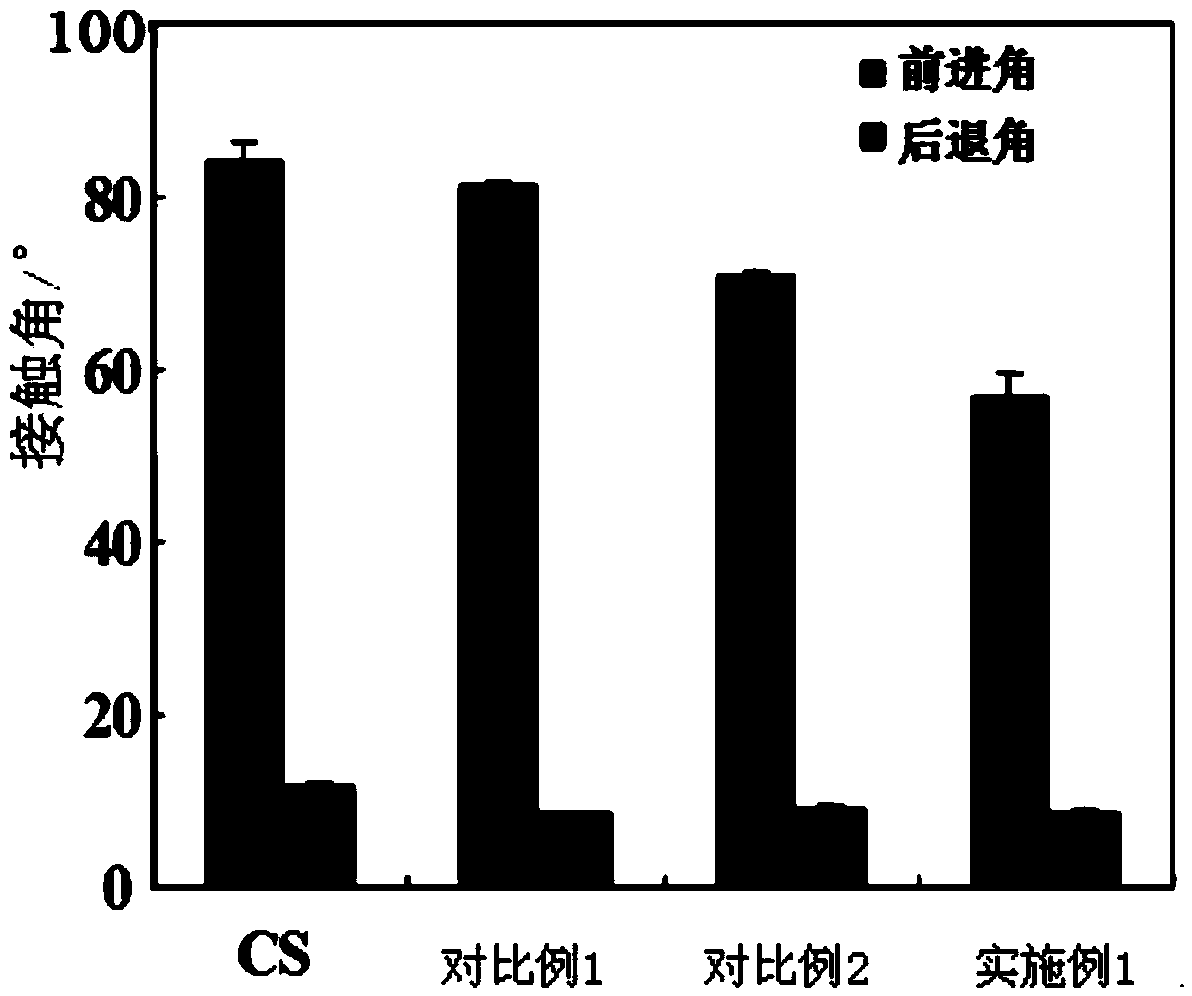
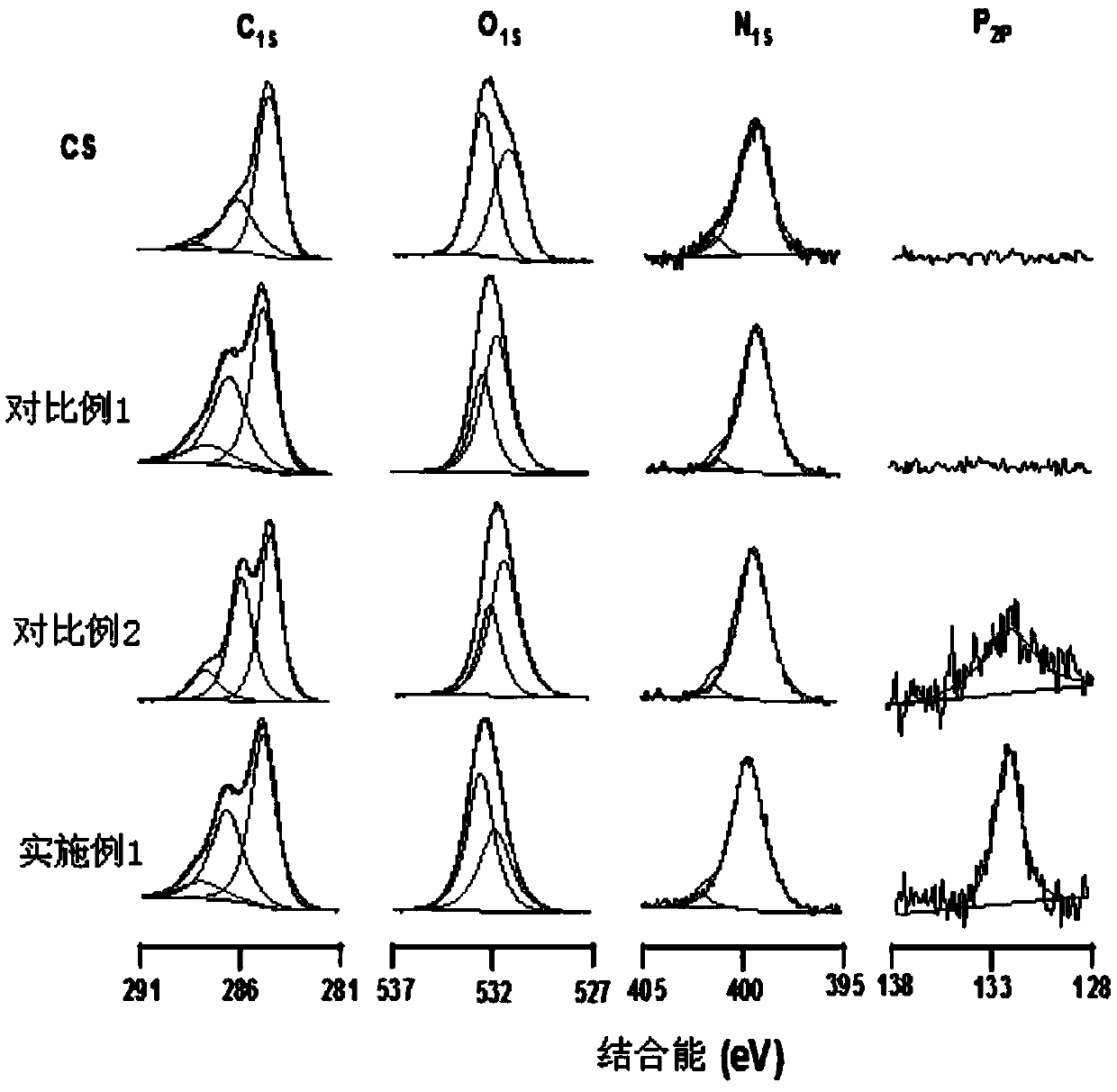
Method for improving chitosan membrane blood compatibility through one-step coating at room temperature
A technology of blood compatibility and chitosan film, applied in the direction of coating, etc., can solve the problems of limited application range, harsh synthesis process conditions, difficult to preserve, etc., achieve broad academic value, good effect of element content and contact angle , Adhesion reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment includes the following steps:
[0031] Step 1. Under the condition of nitrogen protection, 16mmol of 2-methacryloyloxyethyl phosphorylcholine monomer and 4mmol of glycidyl methacrylate monomer are subjected to free radical polymerization reaction under the action of 0.1mmol initiator, free The reaction solvent of the base polymerization reaction is a mixed solvent of ethanol and tetrahydrofuran, the reaction temperature is 70°C, and the reaction time is 24h. After the reaction is completed, it is dialyzed with a dialysis bag with a molecular weight cut-off of 6000D to 8000D, and then freeze-dried at -50°C. Obtain the phosphorylcholine polymer containing epoxy group; The initiator is azobisisobutyronitrile, and the volume ratio of the ethanol and THF is 4:1;
[0032] Step 2, at room temperature (25° C.), dissolve the phosphorylcholine polymer containing epoxy groups in methanol to obtain a polymer solution with a concentration of 1 mg / mL, and then add eth...
Embodiment 2
[0048] This embodiment includes the following steps:
[0049] Step 1. Under the condition of nitrogen protection, 18mmol of 2-methacryloyloxyethyl phosphorylcholine monomer and 2mmol of glycidyl methacrylate monomer are subjected to free radical polymerization reaction under the action of 0.2mmol initiator, free The reaction solvent of the base polymerization reaction is a mixed solvent of ethanol and tetrahydrofuran, the reaction temperature is 65°C, and the reaction time is 24h. After the reaction is completed, it is dialyzed with a dialysis bag with a molecular weight cut-off of 6000D to 8000D, and then freeze-dried at -50°C. Obtain the phosphorylcholine polymer containing epoxy group; The initiator is azobisisobutyronitrile, and the volume ratio of the ethanol to THF is 5:1;
[0050] Step 2. Dissolve the phosphorylcholine polymer containing epoxy groups in methanol at room temperature (20° C.) to obtain a polymer solution with a concentration of 0.5 mg / mL, and then add Et...
Embodiment 3
[0054] This embodiment includes the following steps:
[0055] Step 1. Under the condition of nitrogen protection, 17mmol of 2-methacryloyloxyethyl phosphorylcholine monomer and 3mmol of glycidyl methacrylate monomer are subjected to free radical polymerization reaction under the action of 0.1mmol initiator, free The reaction solvent of the base polymerization reaction is a mixed solvent of ethanol and tetrahydrofuran, the reaction temperature is 75°C, and the reaction time is 20h. After the reaction is completed, it is dialyzed with a dialysis bag with a molecular weight cut-off of 6000D to 8000D, and then freeze-dried at -50°C. Obtain the phosphorylcholine polymer containing epoxy group; The initiator is azobisisobutyronitrile, and the volume ratio of the ethanol to THF is 5:1;
[0056] Step 2. Dissolve the phosphorylcholine polymer containing epoxy groups in ethanol at room temperature (30° C.) to obtain a polymer solution with a concentration of 2 mg / mL, and then add ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com