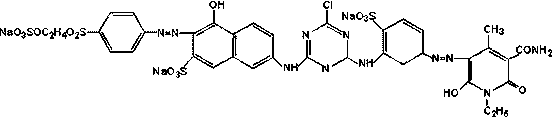
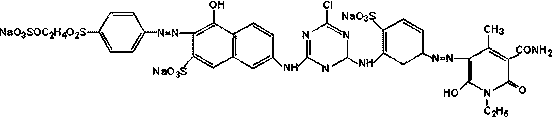
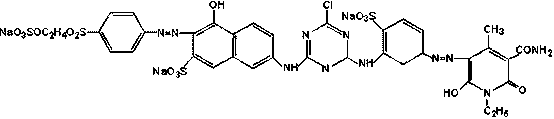
Bright orange reactive dye as well as preparation method and application thereof
A reactive dye and orange technology, applied in reactive dyes, dyeing methods, azo dyes, etc., to achieve good dyeing depth, bright color, and clean process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] Its preparation method comprises the following steps:
[0028] a, a condensation reaction: get cyanuric chloride and disperse it in water to obtain a cyanuric chloride beating liquid, add 2,4-diaminobenzenesulfonic acid aqueous solution in the cyanuric chloride beating liquid, and obtain a primary condensation liquid after the reaction;
[0029] b, diazotization reaction: after adding hydrochloric acid in the said primary condensation liquid, add sodium nitrite again, get diazonium liquid after reaction;
[0030] c. Primary coupling reaction: take N-ethyl-2-pyridone and add it to the diazo solution, and obtain a primary coupling after reaction;
[0031] d. Secondary condensation reaction: take J acid and disperse it in water and add it to the primary coupling compound, and obtain a secondary condensation liquid after the reaction;
[0032] e. Secondary coupling reaction: take the para-ester and disperse it in water, add hydrochloric acid, and then add sodium nitrite to...
Embodiment 1
[0036] 1) Add 100 parts of cyanuric chloride to ice and water for 30 minutes to disperse it in water (cyanuric chloride should be operated below 0°C, otherwise it will be easily decomposed), and 100 parts of 2,4-diaminobenzenesulfonate After the acid is dissolved in water, add it to the cyanuric chloride slurry, adjust the pH to 5.0 with dry baking soda, and react for 3 hours to obtain a condensate;
[0037] 2) Add 250 parts of hydrochloric acid (calculated as HCl) to the primary condensate, cool down to T=2°C, and add 102 parts of sodium nitrite aqueous solution (calculated as sodium nitrite) slowly and dispersedly within 20-25 minutes , maintain the temperature T=2°C, and use sulfamic acid to eliminate excess sodium nitrite after reacting for 2 hours; when the starch potassium iodide test paper shows colorless and Congo red test paper shows blue, the end point is reached, and the diazonium solution is obtained;
[0038] 3) Add 100 parts of N-ethyl-2-pyridone into the diazoni...
Embodiment 2
[0044] 1) Beat 110 parts of cyanuric chloride with appropriate amount of water for 30 minutes, dissolve 100 parts of 2,4-diaminobenzenesulfonic acid and add it to the beating liquid of cyanuric chloride, adjust pH=6.0 with baking soda dry powder, and react A condensate was obtained in 4 hours;
[0045] 2) Add 300 parts of hydrochloric acid (calculated as HCl) to the primary condensate, cool down to 5°C, add 102 parts of sodium nitrite aqueous solution (calculated as sodium nitrite) within 20-25 minutes, and maintain the temperature T= After reacting at 5°C for 2 hours, use sulfamic acid to eliminate excess sodium nitrite; when the starch potassium iodide test paper is used to detect colorless and Congo red test paper to detect blue, the end point is reached, and the diazonium solution is obtained;
[0046] 3) Add 110 parts of N-ethyl-2-pyridone into the diazonium solution obtained in step 2), adjust the pH to 7.0 with baking soda dry powder, maintain the temperature T=10°C, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color fastness | aaaaa | aaaaa |
| color fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com