Single-size CsPbX3 perovskite nanocrystalline preparation method
A technology of perovskite and nanocrystals, which is applied in the field of synthesis of single-size CsPbX3 perovskite nanocrystals, can solve the problems of small size, uncontrollable, and unobtainable, and achieve easy-to-adjust product size, controllable components, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] First, a cesium oleate solution is prepared. Mix 2 mmol (0.648 g) of cesium carbonate powder, 10 mmol (3.35 mL) of OA (oleic acid) and 6.65 mL of ODE (octadecene), heat to 150°C under nitrogen protection to dissolve cesium carbonate, and cool to 100 ℃, prepared into a 0.2M cesium oleate solution, a light yellow transparent solution.
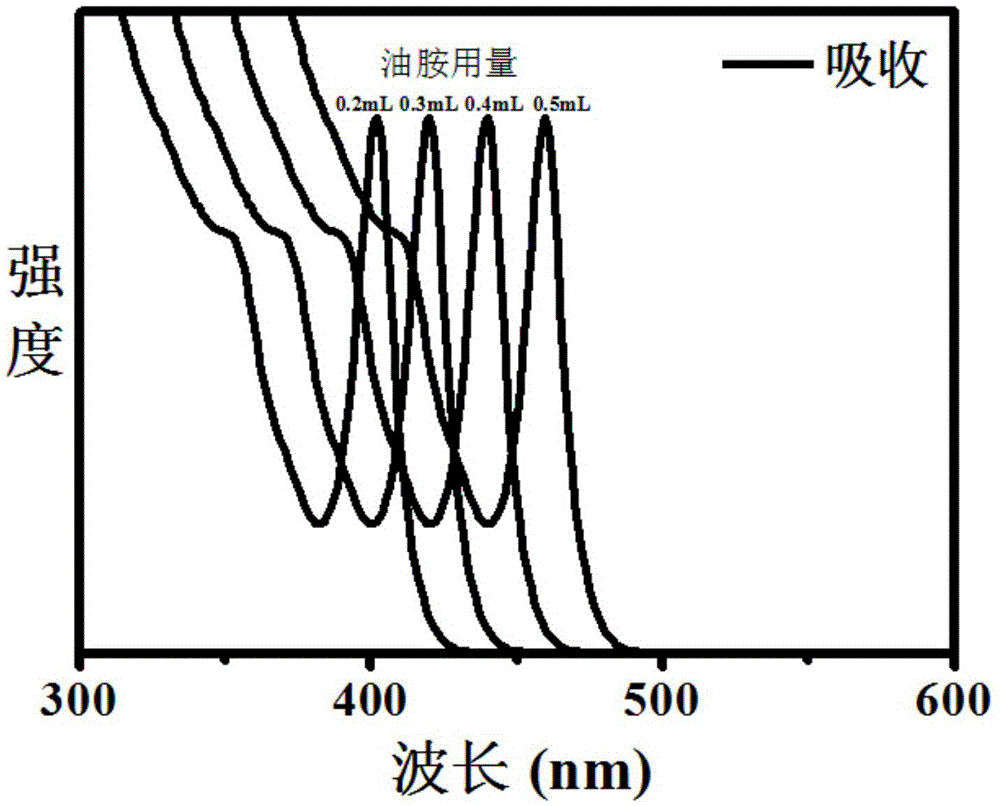
[0026] Then take 0.2 mmol of lead bromide solid powder, 0.2 mL of oleylamine, 0.2 mL of oleic acid and 4 mL of dodecane into a three-necked flask, evacuated at 50°C for 30 minutes, filled with nitrogen for protection, and then heated to 150°C, After the lead bromide was dissolved, it was cooled to room temperature of 25 °C, and then 0.2 mL of 0.2 M cesium oleate in octadecene solution was injected to obtain single-size CsPbBr with an absorption peak at 402 nm. 3 Perovskite Nanocrystals. Its absorption spectrum is shown in figure 1 .
Embodiment 2
[0028] First, a cesium oleate solution is prepared. Mix 2 mmol (0.648 g) of cesium carbonate powder, 10 mmol (3.35 mL) of OA (oleic acid) and 6.65 mL of ODE (octadecene), heat to 150°C under nitrogen protection to dissolve cesium carbonate, and cool to 100 ℃, prepared into a 0.2M cesium oleate solution, a light yellow transparent solution.
[0029] Then take 0.2 mmol of lead bromide solid powder, 0.3 mL of oleylamine, 0.2 mL of oleic acid and 4 mL of dodecane into a three-necked flask, evacuated at 50°C for 30 minutes, filled with nitrogen for protection, and then heated to 150°C, After the lead bromide was dissolved, it was cooled to room temperature of 25 °C, and then 0.2 mL of 0.2 M cesium oleate in octadecene solution was injected to obtain single-size CsPbBr with an absorption peak at 420 nm. 3 Perovskite Nanocrystals. Its absorption spectrum is shown in figure 1 , and its electron microscope photos can be found in figure 2 .
Embodiment 3
[0031] First, a cesium oleate solution is prepared. Mix 2 mmol (0.648 g) of cesium carbonate powder, 10 mmol (3.35 mL) of OA (oleic acid) and 6.65 mL of ODE (octadecene), heat to 150°C under nitrogen protection to dissolve cesium carbonate, and cool to 100 ℃, prepared into a 0.2M cesium oleate solution, a light yellow transparent solution.
[0032]Then take 0.2 mmol of lead bromide solid powder, 0.4 mL of oleylamine, 0.2 mL of oleic acid and 4 mL of dodecane into a three-necked flask, vacuumize at 50°C for 30 minutes, fill with nitrogen for protection, and then heat up to 150°C, After the lead bromide was dissolved, it was cooled to room temperature of 25 °C, and then 0.2 mL of 0.2 M cesium oleate in octadecene solution was injected to obtain a single-size CsPbBr with an absorption peak at 435 nm. 3 Perovskite Nanocrystals. Its absorption spectrum is shown in figure 1 , and its electron microscope photos can be found in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com