Ethylenically double bond-containing trithiocarbonate compound, its preparation method and application
A technology of trithiocarbonate and olefinic double bonds, which is applied in the field of preparation of trithiocarbonate compounds, can solve problems such as complex processing technology and restrictions on material promotion and application, and achieve good reproducibility, good application prospects, Easily Adjustable Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
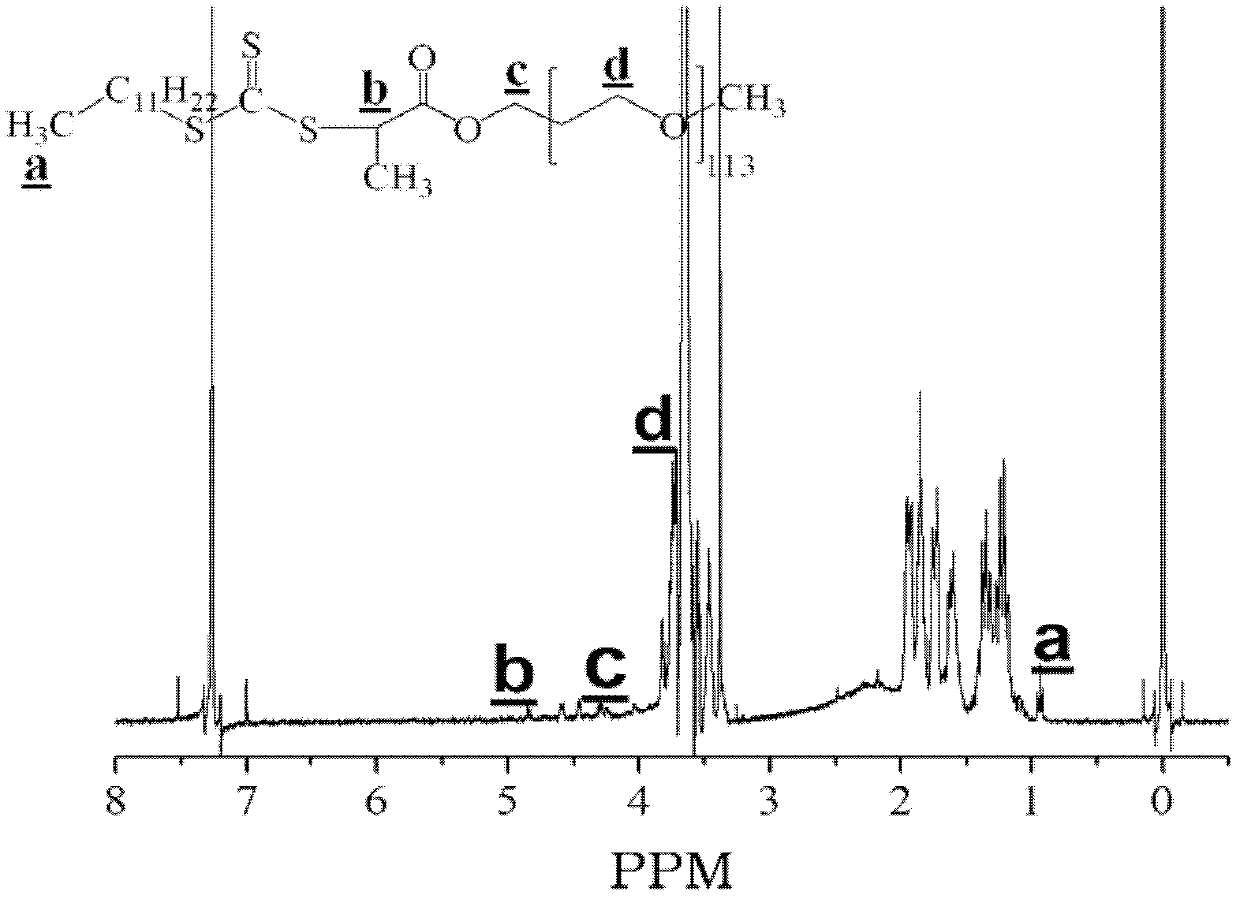
[0055] (1) Preparation of 2-(dodecyltrithiocarbonate)propionic acid.
[0056] Mix 10 grams of sodium hydroxide, 5.4 grams of tetrapropylammonium bromide and 10 grams of water uniformly at room temperature, add them to a 500 mL three-necked round-bottomed flask with a stirring bar and stir, and protect the reaction system with nitrogen gas. 60mL of dodecanethiol was added dropwise into a three-necked round-bottomed flask with a syringe, and the reaction was continued for 0.5 hours after the dropwise addition; During the dropwise addition, it can be observed that white flocs are generated and gradually increase. After the dropwise addition, the reaction is kept for 0.5 hours, and then ice bathed. After the temperature of the material in the reactor was completely lowered to 0°C, 15 mL of carbon disulfide was added dropwise into the flask with a syringe, and the reaction solution turned into a yellow turbid solution at this time. After keeping the reaction for 0.5 hour, 38.42 g ...
Embodiment 2
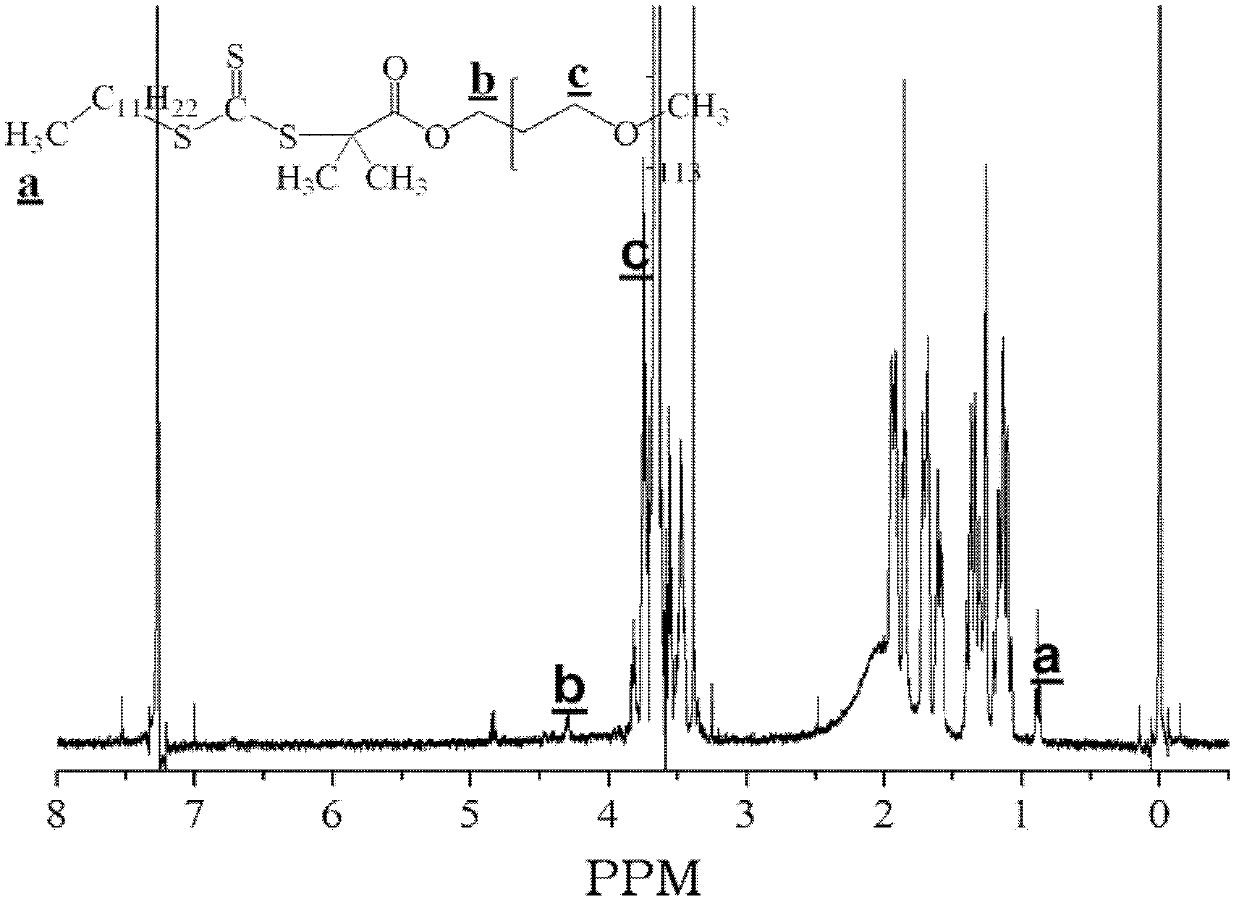
[0063] (1) Preparation of 2-(butanyl trithiocarbonate group) propionic acid
[0064] Mix 10 grams of sodium hydroxide, 5.4 grams of tetrapropylammonium bromide and 10 grams of water uniformly at room temperature, add them to a 500 mL three-necked round-bottomed flask with a stirring bar and stir, and protect the reaction system with nitrogen gas. 27mL of butanethiol was added dropwise into the three-necked round-bottomed flask with a syringe, and the reaction was continued for 0.5 hours after the dropwise addition; During the dropwise addition, it can be observed that white flocs are generated and gradually increase. After the dropwise addition, the reaction is kept for 0.5 hours, and then ice bathed. After the temperature of the material in the reactor was completely lowered to 0°C, 15 mL of carbon disulfide was added dropwise into the flask with a syringe, and the reaction solution turned into a yellow turbid solution at this time. After keeping the reaction for 0.5 hour, 3...
Embodiment 3
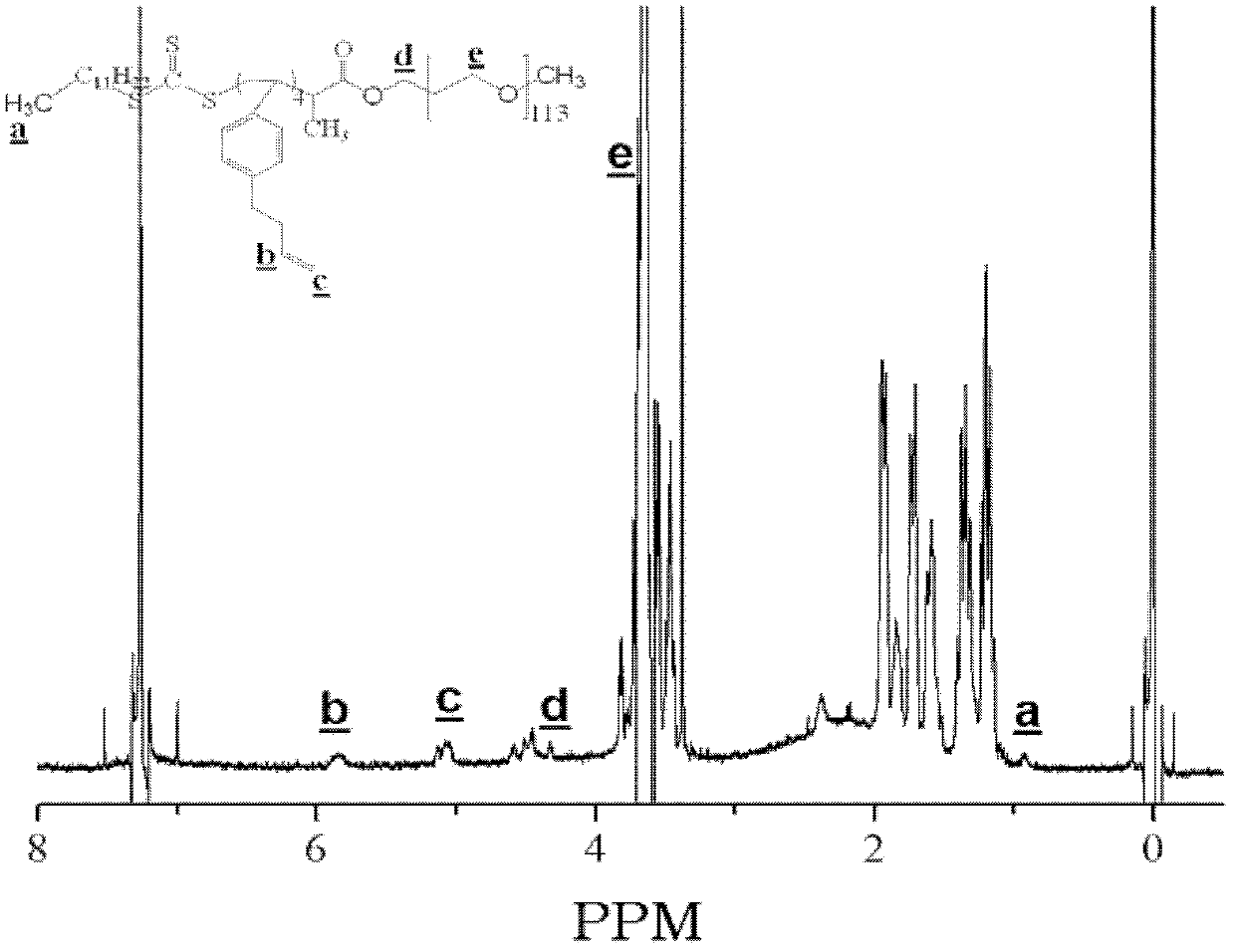
[0071] (1) Preparation of 2-(dodecyl trithiocarbonate group) isobutyric acid
[0072]Add 202.4 grams of dodecyl mercaptan, 16.14 grams of methyl trioctyl ammonium chloride and 482.06 grams of acetone into a 500 mL three-necked flask with a reflux condensing device, the temperature of the water bath is 10 ° C, magnetic stirring, nitrogen, Deoxygenation for 20 minutes; Add 40 grams of aqueous sodium hydroxide solution dropwise (the mass percentage composition of sodium hydroxide is 50%), add in 20 minutes, continue to react for 15 minutes; Add dropwise by 76.1 grams of carbon disulfide and 98.74 grams The mixed solution of acetone composition, after adding in 20 minutes, continue to react 10 minutes; Add the chloroform of 179.07 grams, then add dropwise the sodium hydroxide aqueous solution of 200 grams (the mass percent composition of sodium hydroxide is 50%) , 0.5 hour dropwise is completed, stirring reaction 12 hours; Add 1500 grams of deionized water, then add 109.5 grams of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com