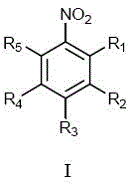
Ligand-free and non-solvent synthesis method for preparing secondary amine under catalysis of ruthenium trichloride
A ruthenium trichloride-catalyzed, synthetic method technology, applied in the field of catalytic chemistry, can solve environmental hazards and other problems, and achieve the effect of avoiding the use of phosphine-containing ligands and organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
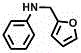
[0025] Example 1: RuCl 3 The catalytic system catalyzes the reaction of nitrobenzene and benzyl alcohol.
[0026]
[0027] Nitrobenzene (1mmol), benzyl alcohol (1.5mmol), K 2 CO 3 (1 mmol), RuCl 3 (3mol%) and glycerol (10mmol) were added to a dry reaction tube with a magnetic stirrer, and then the reaction tube was washed with N 2 Replaced 3 times, stirred and reacted at 130°C for 24h. After the reaction, the solution was cooled to room temperature, 5 mL of water was added, and then extracted with 3×5 mL of chloroform, the organic phase was combined, and the organic phase was dried with anhydrous magnesium sulfate, filtered, and the filtrate was concentrated by rotary evaporation and separated by silica gel column chromatography. , to obtain the target product (yield 80%).
[0028] 1 H-NMR (400MHz, DMSO- d 6 ):δ4.26(d, J =4.0Hz,2H),6.22(t, J =8.0Hz,1H),6.50(t, J =8.0Hz,1H),6.57(d, J =8.0Hz,2H),7.03(t, J =8.0Hz,2H),7.22(t, J =8.0Hz,1H),7.31(t, J =8.0Hz,2H),7.3...
Embodiment 2
[0030] Example 2: RuCl 3 The catalytic system catalyzes the reaction of nitrobenzene and p-methylbenzyl alcohol.
[0031] Nitrobenzene (1mmol), p-methylbenzyl alcohol (1.5mmol), K 2 CO 3 (1 mmol), RuCl 3 (3mol%) and glycerol (10mmol) were added to a dry reaction tube with a magnetic stirrer, and then the reaction tube was washed with N 2 Replaced 3 times, stirred and reacted at 130°C for 24h. After the reaction, the solution was cooled to room temperature, 5 mL of water was added, and then extracted with 3×5 mL of chloroform, the organic phase was combined, and the organic phase was dried with anhydrous magnesium sulfate, filtered, and the filtrate was concentrated by rotary evaporation and separated by silica gel column chromatography. , to obtain the target product (yield 84%, the structural formula is shown below).
[0032]
[0033] 1 H-NMR (400MHz, DMSO- d 6 ):δ2.27(s,3H),4.20(d, J =8.0Hz,2H),6.15(t, J =8.0Hz,1H),6.50(t, J =8.0Hz,1H),6.55(d, J =8.0Hz,2H),7.02(...
Embodiment 3
[0035] Example 3: RuCl 3 The catalytic system catalyzes the reaction of nitrobenzene and p-methoxybenzyl alcohol.
[0036] Nitrobenzene (1mmol), p-methoxybenzyl alcohol (1.5mmol), K 2 CO 3 (1 mmol), RuCl 3 (3mol%) and glycerol (10mmol) were added to a dry reaction tube with a magnetic stirrer, and then the reaction tube was washed with N 2 Replaced 3 times, stirred and reacted at 130°C for 24h. After the reaction, the solution was cooled to room temperature, 5 mL of water was added, and then extracted with 3×5 mL of chloroform, the organic phase was combined, and the organic phase was dried with anhydrous magnesium sulfate, filtered, and the filtrate was concentrated by rotary evaporation and separated by silica gel column chromatography. , to obtain the target product (yield 85%, the structural formula is shown below).
[0037]
[0038] 1 H-NMR (400MHz, DMSO- d 6 ):δ3.71(s,3H),4.17(d, J =4.0Hz,2H),6.12(t, J =8.0Hz,1H),6.49(t, J =8.0Hz,1H),6.55(d, J =8.0Hz,2H),6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com