Novel method for resolving 1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline through enzyme catalysis
A kind of technology of methoxybenzyl and octahydroisoquinoline, which is applied in the field of preparation of key intermediates of antitussives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
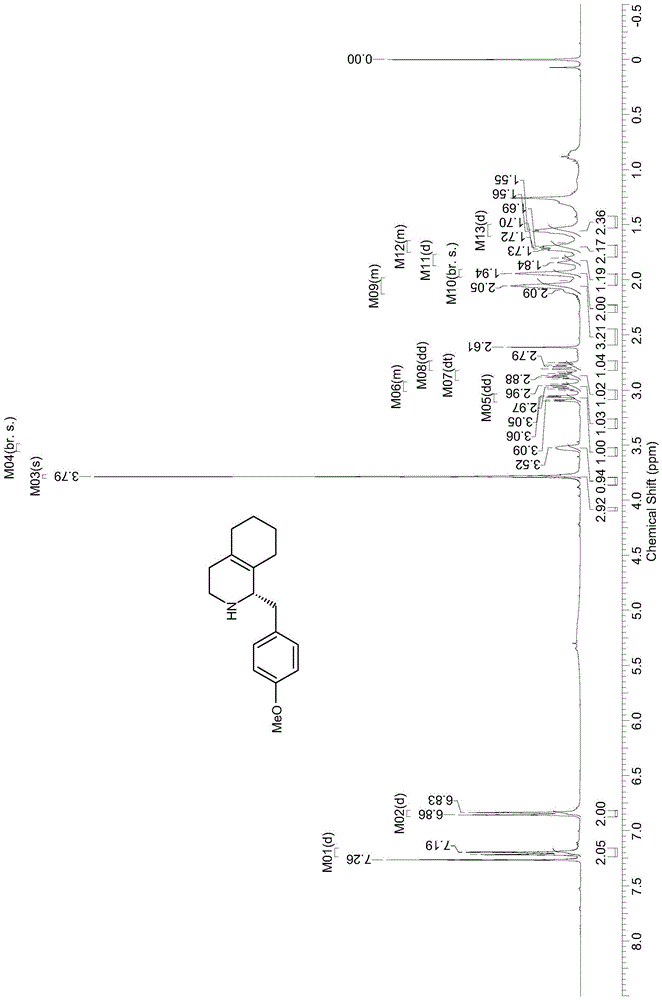
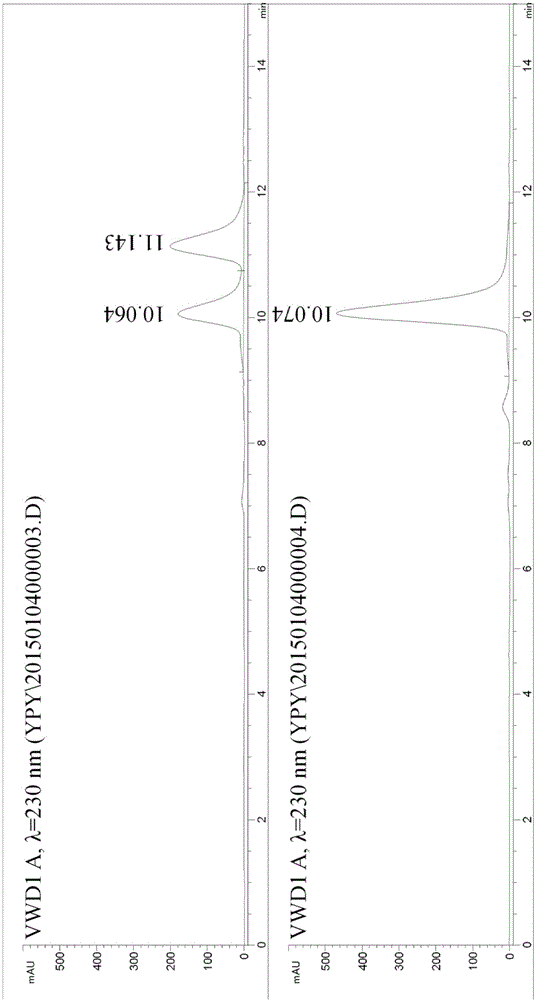
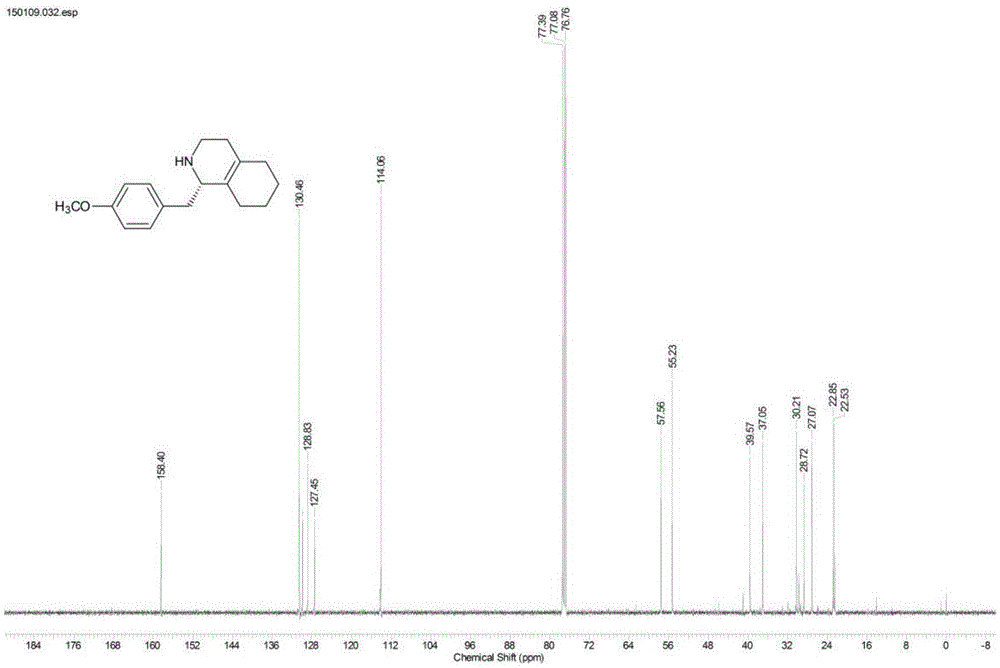
[0022] Example 1: Dynamic kinetic resolution of 1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline
[0023] 1-(4-Methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline (100mg), supernatant of mutant Y321I strain 50mL (take 8g after centrifugation) The wet cells were dissolved in 100mM, pH 6.5 phosphate buffer, high-pressure crushed, centrifuged at 12000×g for 30min to get the supernatant) and ammonium borate (80mg), and the reaction was carried out at 25°C and 200rpm in a shaker. The reaction progress was monitored by liquid chromatography, and the reaction was completed after 32 hours. The pH was adjusted to 10 with 5M sodium hydroxide and extracted three times with dichloromethane (50 mL×3). The extract was concentrated to 25 mL and then mixed with 25 mL of 1M hydrochloric acid solution. The separated aqueous phase was adjusted to pH 10 with 5M sodium hydroxide and extracted three times with dichloromethane (25 mL×3). The organic extract was dried over anhydrous sodiu...
Embodiment 2
[0024] Example 2: Dynamic kinetic resolution of 1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline
[0025] 1-(4-Methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline (129 mg) was dissolved in 1 mL dimethyl sulfoxide, and added to mutant Y321I to destroy bacteria Supernatant 50mL (dissolve 10g of wet bacteria in 100mM, pH6.5 phosphate buffer after expression and centrifugation, and then centrifuge at 12000×g for 30min to get the supernatant) and ammonium boron (124mg), react at 25°C, 200rpm in a shaker. The reaction process was monitored by liquid chromatography. After 20 hours, the reaction was completed, and 6M hydrochloric acid was added to terminate the reaction. The pH was adjusted to 10 with 5M sodium hydroxide, and then extracted three times by high-speed centrifugation with ethyl acetate (50mL×3). After the organic extract was dried over anhydrous sodium sulfate, 100 mg of the product was obtained by separation and purification by column chromatography, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com