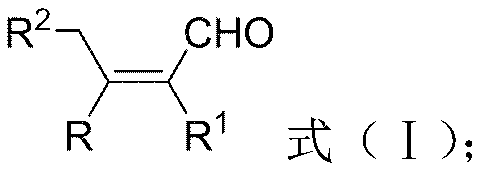
Stereospecific synthesized tetra-substituted olefin compound and novel method therefor
A tetra-substituted and alkene-based technology, which is applied in the field of stereospecific synthesis of tetra-substituted alkene compounds, can solve the problem of not being able to obtain tetra-substituted alkenes, and achieves the effects of high stereoselectivity, mild conditions and easy availability of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
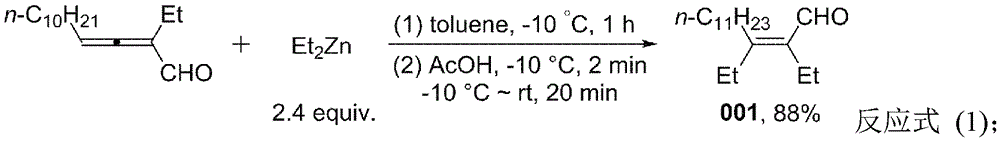
[0024] Embodiment 1 (Z)-2, the synthesis of 3-diethyl-2-tetradecenal (001)
[0025]
[0026] Take a dry Schlenk reaction bottle and pump it under nitrogen three times. Under the protection of nitrogen, add 2-ethyl-2,3-tetradecadienal (0.2358g, 1.0mmol) and toluene (20mL) in turn to the reaction flask, add diethyl Zinc-based solution (1.2mL, 2.0Mintoluene, 2.4mmol), dripped within 4 minutes. The reaction was stirred at -10°C for 1 hour, then acetic acid (2 mL) was added dropwise by injection under stirring at -10°C, and the drop was completed within 2 minutes, and then returned to room temperature. After 20 minutes, add ethyl acetate (20mL), wash with dilute hydrochloric acid (20mL), saturated sodium bicarbonate solution (20mL), and saturated sodium chloride solution (20mL) successively, combine the aqueous phase, and wash the aqueous phase with diethyl ether (20mL× 2) Extract, combine the organic phases, dry over anhydrous sodium sulfate, filter, spin off the solvent and ...
Embodiment 2
[0027] Embodiment 2 (Z)-2, the synthesis of 3-diethyl-2-tridecenal (002)
[0028]
[0029] According to the method described in Example 1, the difference is that the substrates and reagents used are: 2-ethyl-2,3-tridecadienal (0.2221g, 1.0mmol), toluene (20mL), diethyl Zinc solution (1.6mL, 1.5Mintoluene, 2.4mmol) and acetic acid (2mL) gave (Z)-2,3-diethyl-2-tridecenal (0.2273g, 90%) (petroleum ether (30- 60°C) / ethyl acetate=100:1): liquid; 1 HNMR (300MHz, CDCl 3 )δ10.07(s, 1H, CHO), 2.53(t, J=7.8Hz, 2H, CH 2 ), 2.34-2.20 (m, 4H, CH 2 ×2), 1.57-1.42(m, 2H, CH 2 ), 1.41-1.19 (m, 14H, CH 2 ×7), 1.10(t, J=7.5Hz, 3H, CH 3 ), 0.94(t, J=7.7Hz, 3H, CH 3 ), 0.88(t, J=6.8Hz, 3H, CH 3 ); 13 CNMR (75MHz, CDCl 3 )δ191.2, 163.9, 137.7, 31.7, 30.7, 30.0, 29.6, 29.41, 29.39, 29.3, 29.2, 27.3, 22.5, 18.2, 13.9, 12.7; IR (neat) v (cm -1 ) 2961, 2926, 2855, 2752, 1669, 1618, 1464, 1376, 1336, 1287, 1251, 1155, 1060; MS (70ev, EI) m / z (%) 252 (M +, 30.82), 223(100); HRMScalcdforC ...
Embodiment 3
[0030] The synthesis of embodiment 3 (Z)-2-methyl-3-ethyl-2-tridecenal (003)
[0031]
[0032] According to the method described in Example 1, the difference is that the substrates and reagents used are: 2-methyl-2,3-tridecadienal (0.2076g, 1.0mmol), toluene (20mL), diethyl Zinc solution (1.6mL, 1.5Mintoluene, 2.4mmol) and acetic acid (2mL) gave (Z)-2-methyl-3-ethyl-2-tridecenal (0.2181g, 92%) (petroleum ether ( 30-60°C) / ethyl acetate=100:1): liquid; 1 HNMR (300MHz, CDCl 3 )δ10.09(s, 1H, CHO), 2.55(t, J=7.8Hz, 2H, CH 2 ), 2.28(q, J=7.5Hz, 2H, CH 2 ), 1.75 (s, 3H, CH 3 ), 1.58-1.41 (m, 2H, CH 2 ), 1.40-1.17 (m, 14H, CH 2 ×7), 1.08(t, J=7.7Hz, 3H, CH 3 ), 0.88(t, J=6.8Hz, 3H, CH 3 ); 13 CNMR (75MHz, CDCl 3 )δ191.4, 164.5, 131.6, 31.8, 30.5, 30.4, 29.6, 29.5, 29.4, 29.3, 29.2, 28.1, 22.6, 14.0, 11.9, 10.1; IR (neat) v (cm -1 ) 2957, 2925, 2855, 2752, 1671, 1623, 1467, 1396, 1377, 1325, 1282, 1156, 1029; MS (70ev, EI) m / z (%) 238 (M + , 27.08), 43(100); HRMScalcdfor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com