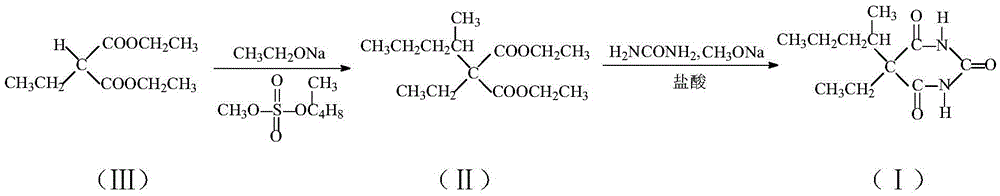
Method for preparing 5-ethyl-5-(1-methylbutyl)malonylurea
A technology of methyl butyl and malonyl urea, which is applied in the field of drug synthesis, can solve the problems of many by-products, long reaction time, and high cost, and achieve the effects of mild reaction conditions, simple post-processing, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Add 453.3g sodium ethoxide ethanol solution (15% content) and an appropriate amount of ethyl acetate into the reaction flask, heat up to 60-70° C. under stirring, and react for 30 minutes. Add 188.2g of diethyl ethyl malonate. After the addition is complete, ethanol is distilled out during the heating reaction process until the internal temperature reaches about 125°C, then the heating is stopped, and then the alcohol is distilled under reduced pressure until there is no effluent, and the internal temperature is 105°C. pressure, keep the negative pressure in the bottle (in the kettle), add 564.6g of toluene, start to add 182.2g of (2-pentyl) methanesulfonate dropwise, and distill the residual ethanol by fractional distillation while adding dropwise, the maximum temperature does not exceed 78°C, The internal temperature rises gradually, the highest is not more than 110°C, and it takes about 3 hours to complete the addition. After the addition, keep the reflux reaction...
Embodiment 2
[0033] (1) Add 420 g of sodium ethoxide ethanol solution (content 17%) and an appropriate amount of ethyl acetate into the reaction flask, heat up to 60-70° C. under stirring, and react for 30 minutes. Add 188.2g of diethyl ethyl malonate. After the addition is complete, ethanol is distilled out during the heating reaction process until the internal temperature reaches about 125°C, then the heating is stopped, and then the alcohol is distilled under reduced pressure until there is no effluent, and the internal temperature is 105°C. pressure, keep the negative pressure in the bottle (in the kettle), add 564.6g of toluene, start to add 236.9g of (2-pentyl) methanesulfonate dropwise, and distill the residual ethanol by fractional distillation while adding dropwise, the maximum temperature does not exceed 78°C, The internal temperature rises gradually, the highest is not more than 110°C, and it takes about 3 hours to complete the addition. After the addition, keep the reflux reacti...
Embodiment 3
[0037](1) Add 346.8 g of sodium ethoxide ethanol solution (20% content) and an appropriate amount of ethyl acetate into the reaction flask, heat up to 60-70° C. under stirring, and react for 30 minutes. Add 188.2g of diethyl ethyl malonate. After the addition is complete, ethanol is distilled out during the heating reaction process until the internal temperature reaches about 125°C, then the heating is stopped, and then the alcohol is distilled under reduced pressure until there is no effluent, and the internal temperature is 105°C. pressure, keep the negative pressure in the bottle (in the kettle), add 564.6g of toluene, start to add 236.9g of (2-pentyl) methanesulfonate dropwise, and distill the residual ethanol by fractional distillation while adding dropwise, the maximum temperature does not exceed 78°C, The internal temperature rises gradually, the highest is not more than 110°C, and it takes about 3 hours to complete the addition. After the addition, keep the reflux react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com