Double-antibody sandwich ELISA kit for detecting E-type enterovirus pathogen
An enterovirus, enzyme-labeled antibody technology, applied in the biological field, to achieve the effect of improving sensitivity and specificity, easy to use and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The construction of embodiment 1E enterovirus structural protein VP1 and VP2 prokaryotic expression recombinant plasmid
[0023] 1. Primer design
[0024] According to the base composition of the target sequence, primers were designed to amplify the VP1 and VP2 genes, and the upstream and downstream contained BamHI and EcoRI restriction sites respectively. The primer sequences are as follows:
[0025] Primers for amplifying the VP1 sequence:
[0026] E-VP1-FCGC GGATCC GAAACAAGCGTGGAGA (BamHI)
[0027] E-VP1-RCCG GAATTC GTACGAGGTGAGGCT (EcoRI)
[0028] Primers for amplifying the VP2 sequence:
[0029] E-VP2-FCGC GGATCC TCTCCGTCAGCAGAAG (BamHI)
[0030] E-VP2-RCCG GAATTC TGATGCAATAGCCCG (EcoRI)
[0031] 2. Gene amplification
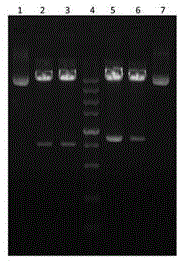
[0032] Genomic RNA of Enterovirus E was extracted by the conventional Trizol method, and cDNA was obtained by using a commercially available conventional reverse transcription kit according to the instructions, and the VP1 gene and VP...
Embodiment 2V
[0037] Expression and purification of embodiment 2VP1 and VP2 recombinant protein
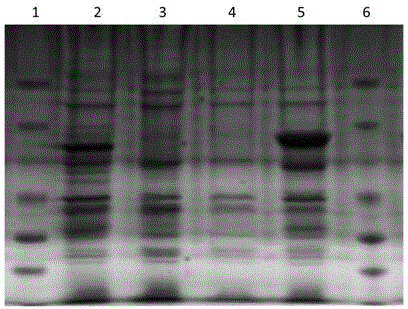
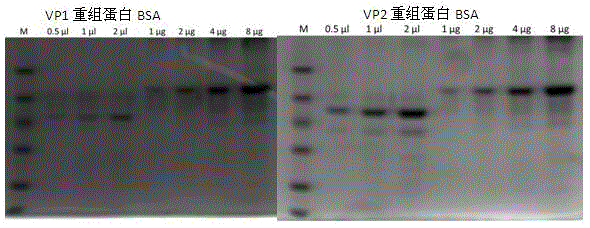
[0038] After transforming the identified positive recombinant plasmids pGEX-4T-1-VP1 and pGEX-4T-1-VP2 into BL21(DE3) competent cells, pick a single colony and inoculate it into 3 mL of LB liquid medium containing 100 μg / mL ampicillin culture overnight, then inoculated 1 mL of the above culture into 200 mL of LB liquid medium containing 100 μg / mL ampicillin and cultured with shaking at 37 °C until the logarithmic growth phase (OD 600 =0.6~0.8), adding IPTG to a final concentration of 1mmol / L, inducing culture at 20°C for 3h, and detecting by SDS-PAGE, the recombinant target proteins VP1 and VP2 were obtained, as shown in figure 2 shown. Centrifuge and precipitate the bacteria, and after sonication, use urea to purify inclusion bodies to obtain high-purity recombinant proteins, such as image 3 shown.
Embodiment 3
[0039] Example 3 Preparation and Purification of Anti-VP1 Mouse Polyclonal Antibody and VP2 Monoclonal Antibody
[0040] 1. Preparation of anti-VP1 mouse-derived polyclonal antibody: select healthy BALB / C mice aged 6-8 weeks, emulsify and purify recombinant VP1 protein with complete Freund's adjuvant, and inject 100 μg subcutaneously at multiple points in each mouse, and then On the 14th day, the same method was used to boost the immunization once, and the serum was collected at the same time to test the titer. For the last booster immunization, 100 μg of purified protein was directly injected intraperitoneally, and blood was collected 3 to 5 days later to collect serum, which was the anti-VP1 mouse polyclonal antibody;
[0041]2. Preparation of anti-VP2 monoclonal antibody: The immunization method is the same as that of anti-VP1 murine polyclonal antibody preparation. Take splenocytes from immunized mice and mix them with SP2 / 0 in a fusion tube, centrifuge at 300g for 10min, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com