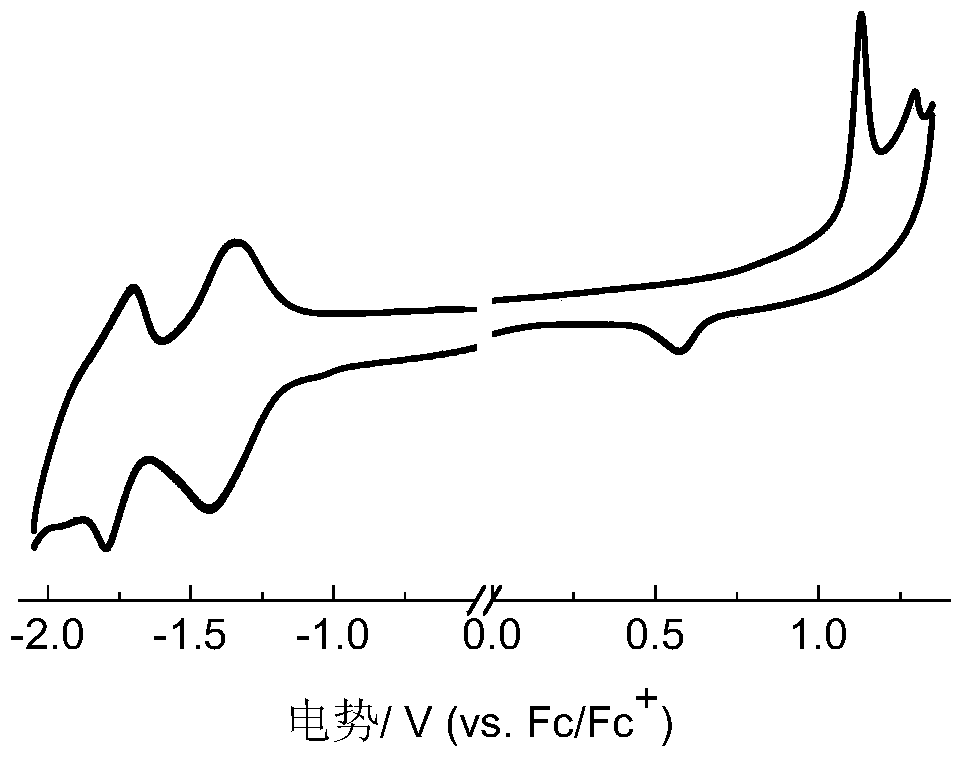
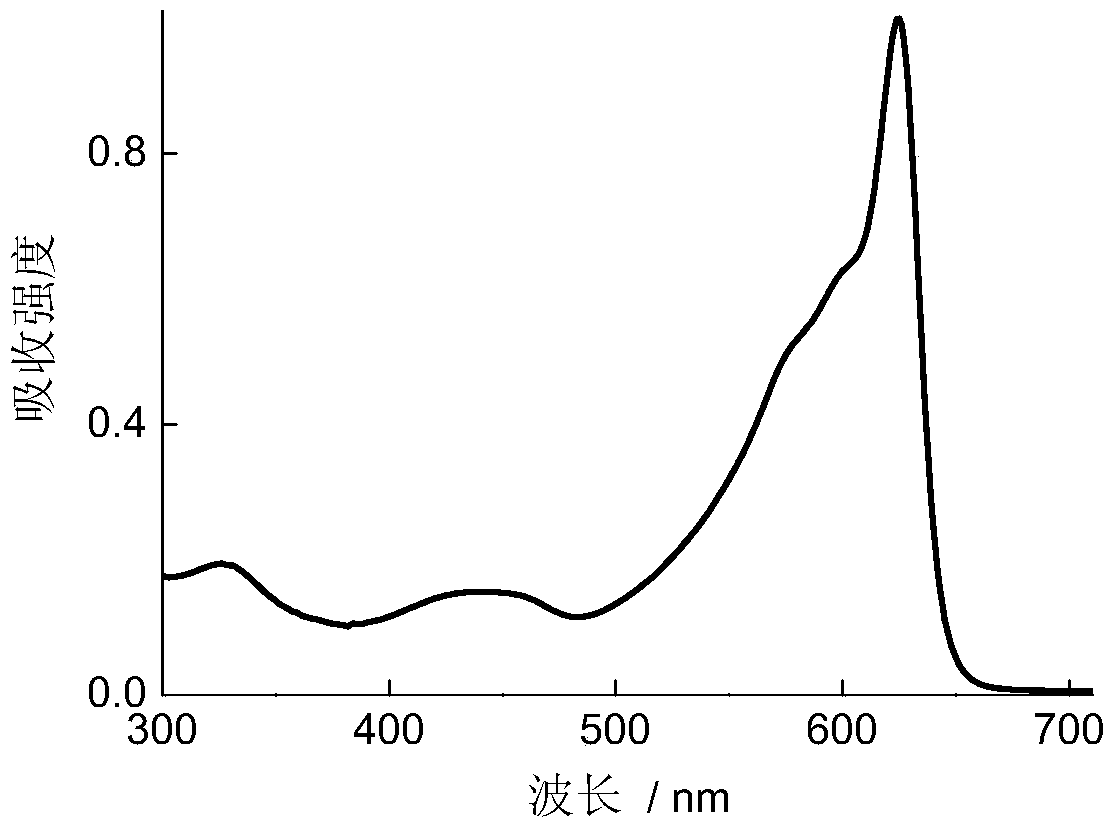
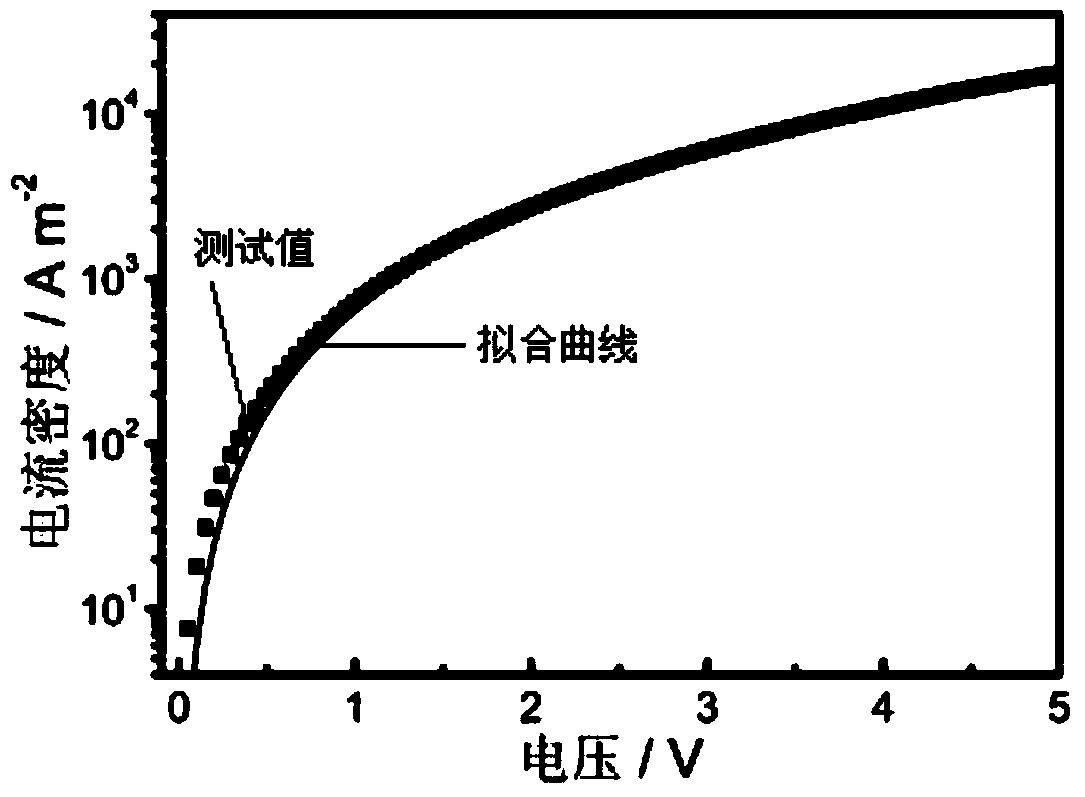
Boron-containing conjugated polymer and its preparation method and application
A technology of conjugated polymer and polymerization reaction, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problem of low energy conversion efficiency of polymer photovoltaic cells, and achieve light enrichment and high photoelectric conversion Efficiency, Effect of Broad Absorption Spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] BNBP-FFBT polymer, the structural formula is as follows (in the structural formula, the capping group is omitted):
[0050]
[0051] The preparation method is: add bis-bromo-BNBP (135.6mg, 0.14mmol), 3,3'-difluoro-2,2'-bithiophene bistin salt (71.6mg, 0.14 mmol), tris(dibenzylideneacetone) dipalladium (2.8mg, 0.003mmol) and tris(o-methyl)phenylphosphine (6.6mg, 0.022mmol), then evacuate the system with argon gas Added distilled toluene solvent (60mL) several times in the dark, and refluxed at 115°C for 48h, then added phenylboronic acid (100mg, 0.82mmol) and continued to reflux for 3h, then added bromobenzene (200mg, 1.28mmol) and refluxed for 3h. The reaction system was cooled to room temperature, dissolved in 100 ml of chloroform, washed with water, dried, and most of the solvent was removed, and the remaining solution was dropped in acetonitrile, and the polymer was precipitated, and the precipitate was sequentially washed with acetone, n-hexane, Tetrahydrofuran ...
Embodiment 2
[0056] BNBP-BFT polymer, the structural formula is as follows (in the structural formula, the capping group is omitted):
[0057]
[0058] The preparation method is: add dibromo-BNBP (150mg, 0.17mmol), 3,4-difluorothiophene bistin salt (75.25mg, 0.17mmol), three (dibenzylidene Indaneacetone) dipalladium (3.5mg, 0.003mmol) and tri(o-methyl)phenylphosphine (8.3mg, 0.027mmol), then vacuumize the system with argon gas for several times, and add Distilled toluene solvent (10mL), after reflux at 115°C for 48h, phenylboronic acid (100mg, 0.82mmol) was added to reflux for 3h, then bromobenzene (200mg, 1.28mmol) was added to reflux for 3h. The reaction system was cooled to room temperature, dissolved in 100 ml of chloroform, washed with water, dried, and most of the solvent was removed, and the remaining solution was dropped in acetonitrile, and the polymer was precipitated, and the precipitate was sequentially washed with acetone, n-hexane, THF washes away small molecules and cata...
Embodiment 3
[0062] BNBPP-2BFT polymer, the structural formula is as follows (in the structural formula, the capping group is omitted):
[0063]
[0064] The preparation method is as follows: add bisbromo-BNBP (180mg, 0.17mmol), difluorobithiophene bistin salt (88mg, 0.17mmol), tris(dibenzylideneacetone) to a clean polymerization bottle after baking Dipalladium (3.5mg, 0.003mmol) and tri(o-methyl)phenylphosphine (8.3mg, 0.027mmol), the system was evacuated several times, and distilled toluene solvent (9mL) was added under the dark state, 115 After reflux at ℃ for 48h, add phenylboronic acid (100mg, 0.82mmol) to reflux for 3h, then add bromobenzene (200mg, 1.28mmol) to reflux for 3h. The reaction system was cooled to room temperature, dissolved in 100 ml of chloroform, washed with water, dried, and most of the solvent was removed, and the remaining solution was dropped in acetonitrile, and the polymer was precipitated, and the precipitate was sequentially washed with acetone, n-hexane, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com