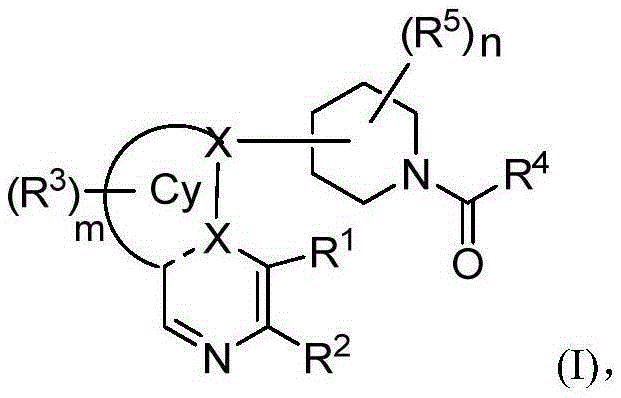
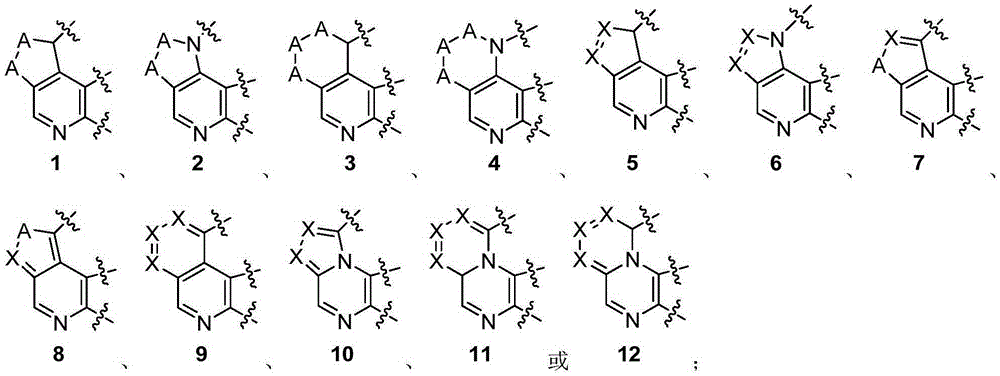
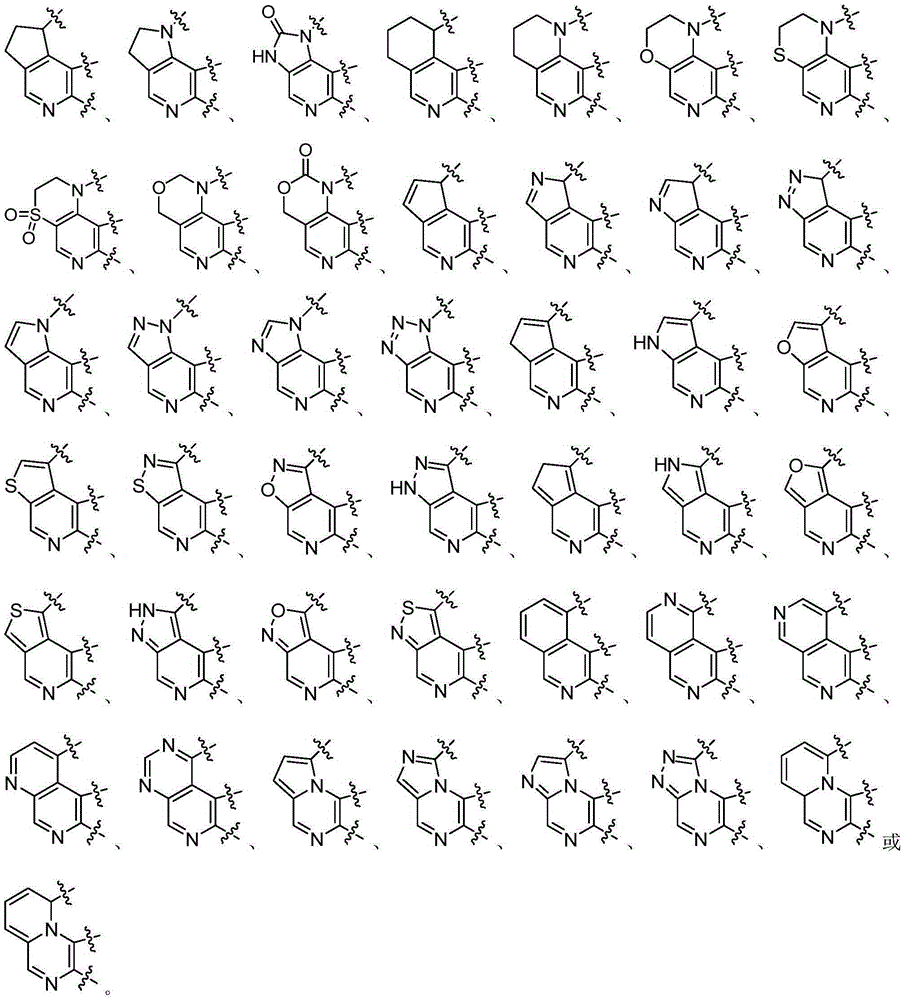
Heterarylation compound and application thereof to drugs
A technology of compounds and hydrates, applied in the field of medicine, can solve problems such as signal conduction disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0257] Example 1N-(2,6-dichloro-4-cyanophenyl)-1H-pyrrolo[2,3-b]pyridine-4-carboxamide
[0258]
[0259] Under ice-cooling, add oxalyl chloride (391.0mg, 3.08mmol) to a solution of 1H-pyrrolo[2,3-b]pyridine-4-carboxylic acid (100.0mg, 0.62mmol) in dichloromethane (5mL), 2 drops DMF was used as a catalyst, and the reaction was heated at 40°C for 2h. The reaction solution was concentrated, and the resulting residue was dissolved in DMF (5 mL), and 4-amino-3,5-dichlorobenzonitrile (173.0 mg, 0.93 mmol) and sodium hydride (60%, 74.0 mg, 1.85mmol) in DMF (5mL) solution, react at room temperature for 12h. After completion of the reaction, water (20 mL) was added to the reaction solution to quench the reaction, extracted with dichloromethane (30 mL × 3), and the combined organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, and concentrate under reduced pressure, and the resulting residue is separated by silica gel column chromatography (eluent: CH 2 Cl 2 / MeOH (v / v)...
Embodiment 2
[0262] Example 23-(4-methyl-3-(methyl(6-(methylamino)pyridazin-4-yl)amino)piperidin-1-yl)-3-oxopropionitrile
[0263]
[0264] Step 1: Compound N 3 ,N 5 -Synthesis of dimethylpyridazine-3,5-diamine
[0265] At room temperature, add 3,5-dichloropyridazine (1.20g, 8.1mmol) and methylamine aqueous solution (10mL) into a 25mL digestion tank, seal it; react in an oil bath at 150°C for 22h, cool to room temperature, and the reaction solution Concentrate directly, and the residue is separated by column chromatography (eluent: CH 2 Cl 2 / MeOH (v / v)=6 / 1), to obtain 1.1 g of light yellow solid, yield: 98%.
[0266] MS(ESI,pos.ion)m / z:139.1[M+1] + .
[0267] Step 2: Compound N 5 -(1-Benzyl-4-methylpiperidin-3-yl)-N 3 ,N 5 -Synthesis of dimethylpyridazine-3,5-diamine
[0268] At room temperature, N,N-dimethylformamide (4 mL) was added to N 3 ,N 5 - In a mixture of dimethylpyridazine-3,5-diamine (146mg, 1.06mmol) and sodium hydride (60%, 84.8mg, 2.12mmol), react at 50°C for ...
Embodiment 33
[0274] Example 33-((3R,4R)-3-((6-amino-5-methylpyrimidin-4-yl)(methyl)amino)-4-methylpiperidin-1-yl)-3- Oxopropionitrile
[0275]
[0276] Step 1: Synthesis of the compound N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-6-chloro-N,5-dimethylpyrimidin-4-amine
[0277] 4,6-dichloro-5-methylpyrimidine (0.60g, 3.68mmol) and (3R,4R)-1-benzyl-N,4-dimethylpiperidin-3-amine (0.80g, 3.68 mmol) was dissolved in DMF (5 mL), potassium carbonate (1.02 g, 7.36 mmol) was added, and the reaction was stirred overnight at 70° C. under nitrogen protection. Add water (100mL) to quench the reaction, extract with ethyl acetate (70mL×3), wash the organic phase with water (50mL), wash with saturated aqueous sodium chloride solution (50mL), dry the organic layer with anhydrous sodium sulfate, concentrate under reduced pressure, and carry out Separation by column chromatography (eluent: PE / EtOAc (v / v) = 1 / 1) to obtain 1.08 g of product, yield: 85.0%.
[0278] MS(ESI,pos.ion)m / z:345.3[M+1] + .
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com