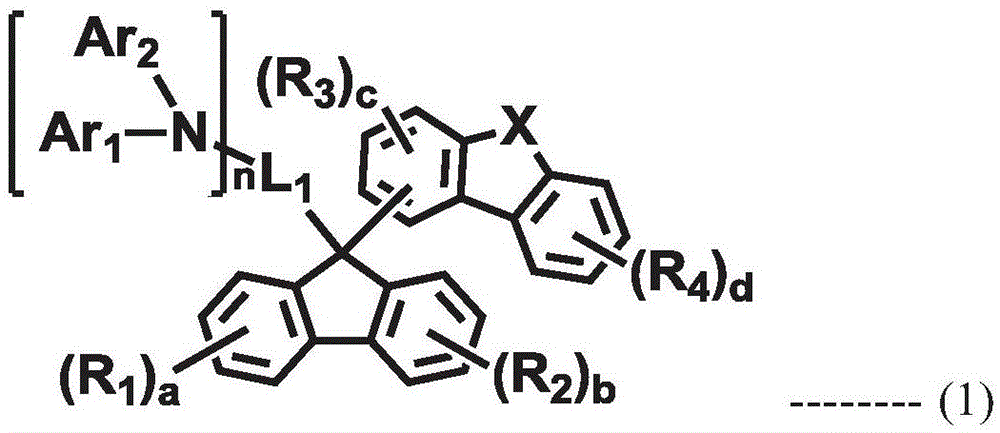
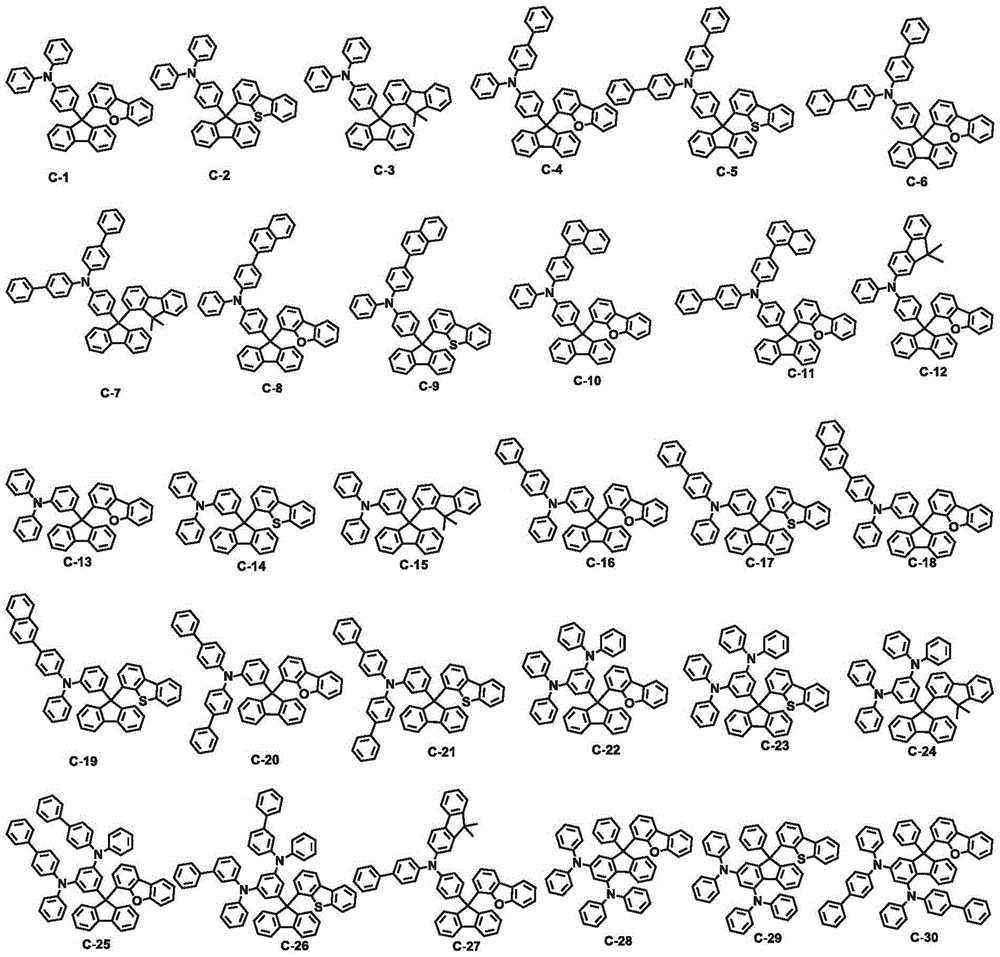
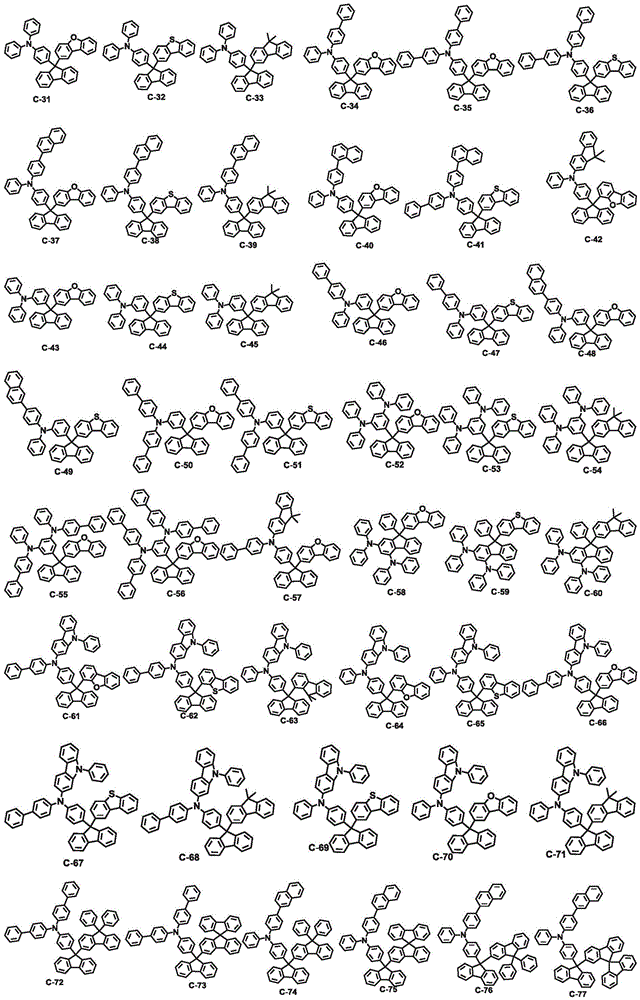
An organic electroluminescent compound and an organic electroluminescent device comprising the same
A technology of luminescence and compounds, which is applied in the field of organic electroluminescent compounds, can solve the problems of reduced device life, non-disclosure of fluorene, poor thermal stability, etc., and achieve excellent current and power efficiency and long life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0107] Example 1: Preparation of Compound C-6
[0108]
[0109] Preparation of compound 1-1
[0110] After introducing dibenzofuran (30 g, 178 mmol) and 500 mL of tetrahydrofuran into the reaction vessel, the vessel was cooled to -78°C under nitrogen atmosphere. Then 71 mL of n-butyllithium (2.5M, 178 mmol) was slowly added dropwise to the mixture. After stirring the mixture at -78°C for 30 minutes, it was stirred at room temperature for 3 hours and cooled to -78°C. Thereafter, fluorenone (32 g, 178 mmol) dissolved in 500 mL of tetrahydrofuran was slowly added dropwise to the mixture. After the addition, the reaction temperature was allowed to warm slowly to room temperature, and the mixture was stirred for 16 hours. Aqueous ammonium chloride solution was then added to the reaction solution to complete the reaction, and the reaction solution was extracted with ethyl acetate. The extracted organic layer was then dried using magnesium sulfate, and the solvent was remov...
example 2
[0118] Example 2: Preparation of Compound C-8
[0119]
[0120] Preparation of compound 2-2
[0121] After compound 2-1 (30 g, 86.1 mmol), 4-bromotriphenylamine (84 g, 259 mmol) and 600 mL of dichloromethane (MC) were introduced into the reaction vessel, the vessel was introduced into a nitrogen atmosphere. Thereafter, 3 mL of Eaton's reagent was slowly added dropwise to the mixture. After the mixture was stirred at room temperature for 2 hours, ethanol and distilled water were added thereto to complete the reaction, and the mixture was extracted with dichloromethane. The extracted organic layer was then dried using magnesium sulfate, and the solvent was removed using a rotary evaporator. The remaining material was then purified by column chromatography to obtain compound 2-2 (42 g, 74%).
[0122] Preparation of Compound C-8
[0123] Compound 2-2 (10g, 26.9mmol), 2-naphthylboronic acid (3.2g, 18.4mmol), tetrakis (triphenylphosphine) palladium (0.7g, 1.08mmol), pot...
example 3
[0125] Example 3: Preparation of Compound C-38
[0126]
[0127] Preparation of compound 3-1
[0128] After introducing 2-bromodibenzothiophene (27 g, 103 mmol) and 340 mL of tetrahydrofuran into the reaction vessel, the vessel was cooled to -78°C under nitrogen atmosphere. Then 33 mL of n-butyllithium (2.5M, 82 mmol) was slowly added dropwise to the mixture. After stirring the mixture at -78°C for 2 hours, 9H-fluoren-9-one (19 g, 103 mmol) dissolved in 340 mL of tetrahydrofuran was slowly added dropwise to the mixture. After the addition, the reaction temperature was allowed to warm slowly to room temperature, and the mixture was stirred for 30 minutes. Aqueous ammonium chloride solution was then added to the reaction solution to complete the reaction, and the mixture was extracted with ethyl acetate. The extracted organic layer was then dried using magnesium sulfate, and the solvent was removed using a rotary evaporator. The remaining material was then purified by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com