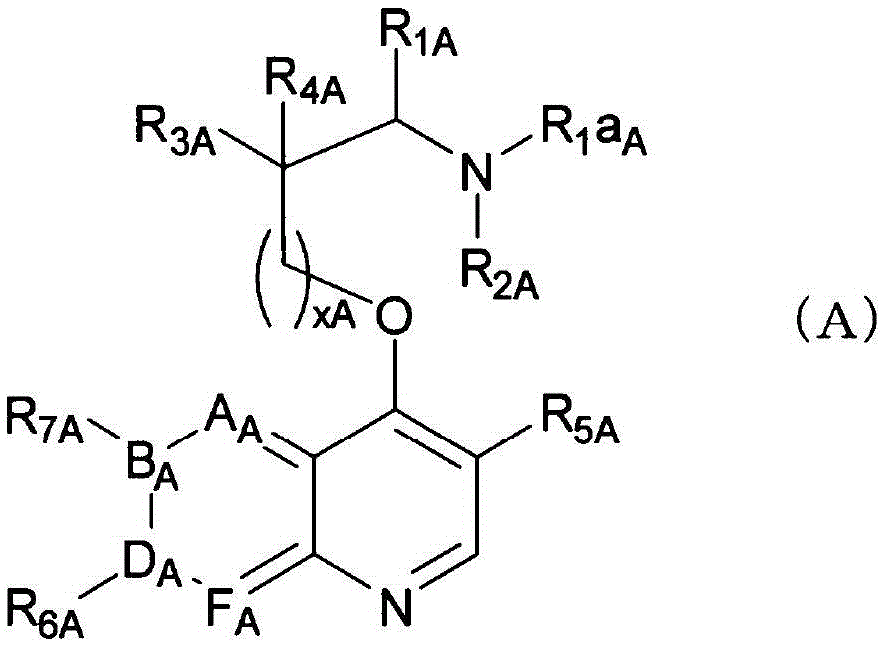
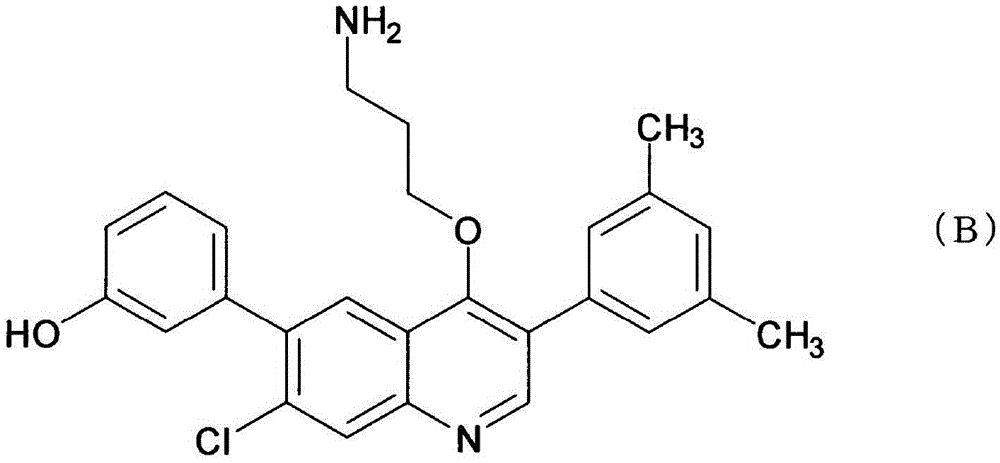
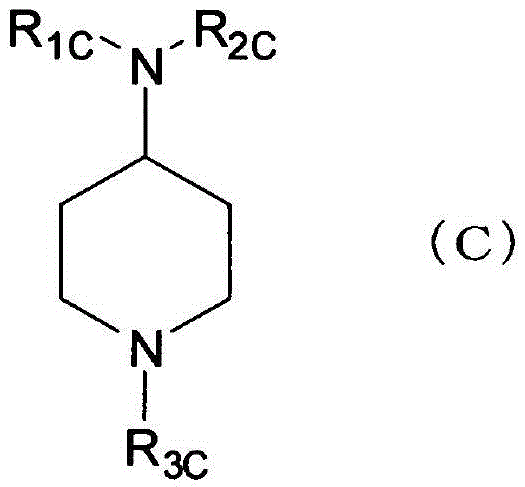
Compound having agonistic activity to somatostatin receptor and medicinal use thereof
A compound and drug technology, applied in the field of compounds with somatostatin receptor agonistic activity and their pharmaceutical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0472] The present invention is specifically described below by way of examples and biological examples, which do not limit the present invention. Compounds of the invention and compounds described in the Examples were named using ACD / Name (version 6.00, available from Advanced Chemistry Development Inc.) or Chemdraw Ultra (version 12.0, available from CambridgeSoft).
[0473] Solvents stated in parentheses in the sections of chromatographic separation and TLC refer to elution solvents or developing solvents used and ratios are represented by volume ratios. For "NH silica gel" is meant the use of CHROMATOREX NHTLCPLATE (catalogue number: 3800003) available from Fuji Silysia Chemical Ltd.
[0474] "Hi-flashSI" and "Hi-flashNH" mentioned in parentheses in the medium pressure preparative liquid chromatography section indicate the type of column used, respectively (Hi-flashSI: silica gel (available from Yamazen Corporation) and Hi-flashNH: aminopropyl Bonded silica gel (available...
Embodiment 2
[0531] tert-Butyl N-{1-[3-(3,5-dichlorophenyl)-5-nitro-4-pyridyl]-4-piperidinyl}carbamate
[0532]
[0533] To a solution of the compound (128.35 g) prepared in Reference Example 1 in 1,4-dioxane (1283 mL) was added 2M aqueous tripotassium phosphate (39.72 mL), 3,5-dichlorophenylboronic acid (CAS# 67492-50-6) (64.08 g) and tetrakis(triphenylphosphine)palladium(0) (1.84 g) and the mixture was stirred at 100° C. for 5 hours. The reaction solution was cooled to room temperature, diluted with ethyl acetate and washed with water and saturated sodium chloride solution. After drying, the organic layer was concentrated to obtain a residue (168 g) containing the title compound having the following physical properties. The resulting residue was used in the next reaction without purification.
[0534] Properties: yellow powder;
[0535] TLC (Rf): 0.40 (n-hexane: ethyl acetate=4:1);
[0536] MASS(ESI,Pos.):467(M+H) + .
[0537] Reference example 3:
[0538] tert-butyl N-{1-[3-am...
Embodiment 1
[0557] 1-{3-(3,5-Dichlorophenyl)-5-[4-(trifluoromethyl)phenyl]-4-pyridyl}-4-piperidinamine
[0558]
[0559] The compound of the present invention having the following physical properties was obtained by the same operation as in Reference Example 2→Reference Example B1, using the compound prepared in Reference Example 4 instead of the compound prepared in Reference Example 1, and using 4-(three Fluoromethyl)phenylboronic acid (CAS# 128796-39-4) was substituted for 3,5-dichlorophenylboronic acid.
[0560] Properties: white powder;
[0561] Purity (UPLC-MS / ELSD): 99.9% (retention time: 0.58min.);
[0562] TLC (Rf): 0.71 (ethyl acetate:methanol=9:1, NH silica gel);
[0563]NMR (300MHz, methanol-d4): δ8.20(s, 1H), 8.19(s, 1H), 7.80(d, J=8.1Hz, 1H), 7.62(d, J=8.1Hz, 1H), 7.53 -7.50(m, 1H), 7.43(d, J=1.8Hz, 2H), 2.91(br.d, J=13.0Hz, 2H), 2.52-2.35(m, 3H), 1.49-1.37(m, 2H ), 1.06-0.88 (m, 2H);
[0564] MASS(ESI,Pos.):466(M+H) + .
[0565] Example 2:
[0566] 1-{3-(3-Chloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com