Preparation method of acifluorfen
A technology of acifluorfen and compounds, which is applied in the field of preparation of acifluorfen, and can solve the problems of harsh synthetic conditions and low yield of acifluorfen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
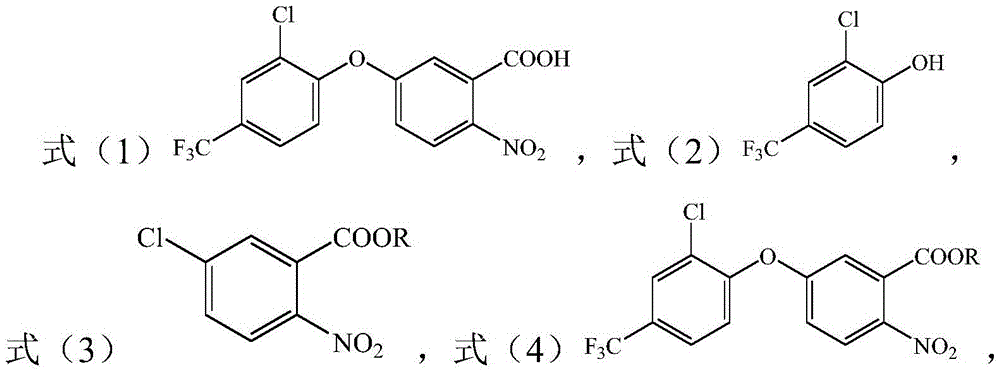
[0014] The present invention provides a method for preparing acifluorfen, wherein the acifluorfen is a compound represented by formula (1), wherein the method comprises:
[0015] (1) drop the compound shown in formula (2) in the organic solvent containing alkali metal carbonate and the compound shown in formula (3), make the compound shown in formula (3) and formula (2) show The compound carries out contact reaction, to make the compound shown in formula (4);
[0016] (2) Alkali hydrolysis and acidification of the compound shown in formula (4) to prepare the compound shown in formula (1);
[0017] Formula 1) Formula (2)
[0018] Formula (3) Formula (4)
[0019] Wherein, R is a C2-C6 linear or branched alkyl group.
[0020] According to the present invention, the present invention ingeniously adopts the method of dripping the compound shown in formula (2) so that the compound shown in formula (2) can directly carry out contact reaction with the compound shown in form...
preparation example 1
[0051] 1) Add isopropanol (92g, 1.5mol) dropwise to m-chlorobenzoyl chloride (175g, 1mol) at 80°C. Introduce nitrogen to drive out HCl gas, then remove isopropanol under negative pressure, and keep it at -0.095MPa, 90°C for 2h, and cool down to obtain 198.4g of isopropyl m-chlorobenzoate with a yield of 98.9%.
[0052] 2) Add isopropyl m-chlorobenzoate (150g, 0.748mol) dropwise to fuming nitric acid (480.6g, 7.48mol) at -10°C, and stir at 25°C after the addition is complete (about 3h) After the reaction was completed for 1 h, the reaction product was poured into 960 g of ice water, stirred for 0.5 h, then suction-filtered and the resulting solid was washed with ethanol and then suction-filtered to obtain 141.5 g of the compound shown in formula (3-3). The yield is 78.6%, and the purity is 96%.
preparation example 2
[0054] According to the method described in Preparation Example 1, the difference is that in step 1), ethanol was used instead of isopropanol to obtain 161.4 g of the compound represented by formula (3-1), with a yield of 70.2% and a purity of 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com