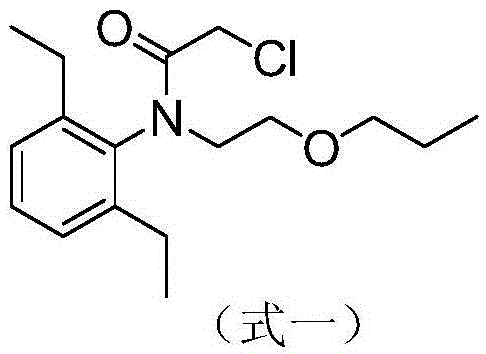
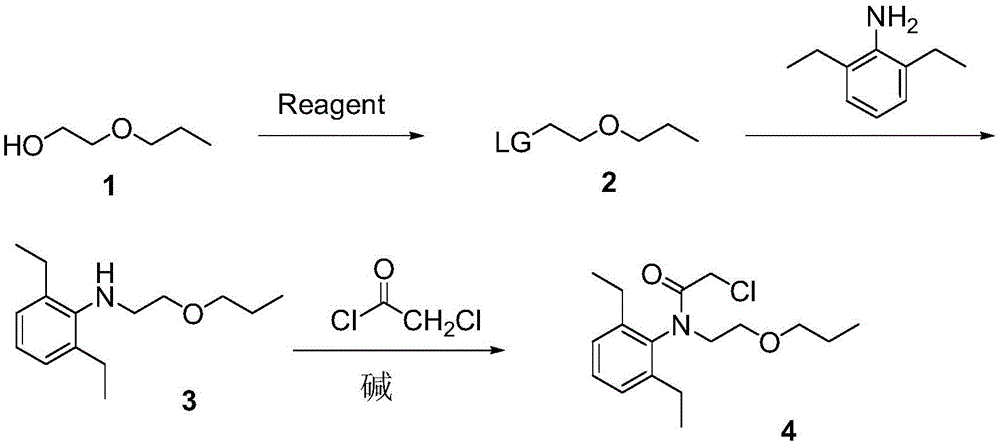
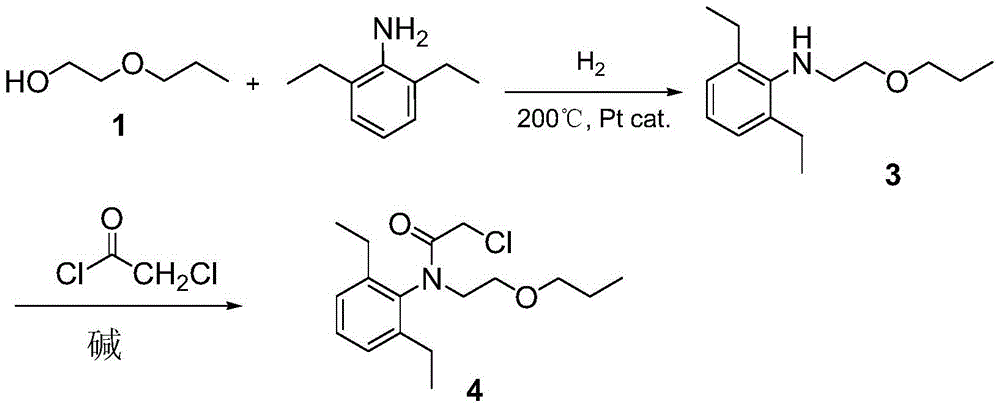
Pretilachlor synthesis method
A synthesis method and a technology of pretilachlor, applied in the field of chemistry, can solve the problems such as the inability to reuse the catalyst repeatedly, expensive platinum catalyst, etc., and achieve the effects of mild conditions, environmental friendliness and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) In a 500ml three-necked flask, add 160mL of n-propanol and 30g (0.365mol) of sodium n-propoxide, stir, and after the sodium n-propoxide is completely dissolved, add 65mL (0.571mol) dropwise (30 minutes) 2-Chloroacetaldehyde dimethyl acetal, the temperature was raised to reflux after dropping, and the reaction was stirred for 6h. After cooling and filtering, the filtrate is distilled at normal pressure to recover n-propanol, and then distilled under reduced pressure by water pump to collect fractions at 70-80°C (-0.098MPa) to obtain the product - propoxyacetaldehyde dimethyl acetal (colorless liquid) 60.6 g, yield 81.9%, detected by GC, the content is 95%.
[0068] (2) In a 1000ml three-necked flask, add 10g of propoxyacetaldehyde dimethyl acetal (0.0641mol) and THF 40mL, cool down in an ice-salt bath to below 0°C, and dropwise add about 25mL of 5M hydrochloric acid solution (10 minutes to complete) (thereby adjusting the pH to be less than 1), after the dropwise ad...
Embodiment 2
[0072] (1) In a 500ml three-neck flask, add 160mL of toluene and 30g (0.365mol) of sodium n-propoxide, stir, add dropwise (30 minutes after the addition) 65mL (0.571mol) of 2-chloroacetaldehyde dimethyl acetal, dropwise The temperature was raised to reflux, and the reaction was stirred for 6h. After cooling and filtering, the filtrate was distilled at normal pressure to recover toluene first, then distilled under reduced pressure by a water pump, and the fraction at 70-80°C (-0.098MPa) was collected to obtain 56.8g of a colorless liquid product with a yield of 76.7%. The content was 94.3% by GC detection. .
[0073] (2) In a 1000ml three-neck flask, add 15g (0.0954mol) of propoxyacetaldehyde dimethyl acetal and THF60mL, cool down in an ice-salt bath to below 0°C, and dropwise add about 40mL of 5M sulfuric acid solution (dropping is completed in 20 minutes) (thereby adjusting the pH to be less than 1), after the dropwise addition was completed, the reaction was stirred at room...
Embodiment 3
[0077] (1) In a 500ml three-necked flask, add 250mL (2.2mol) of 2-chloroacetaldehyde dimethyl acetal, slowly add (30 minutes) 30g (0.365mol) sodium n-propoxide, stir, and heat up to 50~ 60 ° C, stirring the reaction for 6h. After cooling and filtering, the filtrate was distilled at normal pressure to recover unreacted 2-chloroacetaldehyde dimethyl acetal, and then distilled under reduced pressure by a water pump to collect fractions at 70-80°C (-0.098MPa) to obtain 45g of the product colorless liquid with a yield of 60.8 %, detected by GC, the content is 96.0%.
[0078] (2) In a 1000ml three-neck flask, add 15g (0.0972mol) propoxyacetaldehyde dimethyl acetal and THF 60mL, cool down in an ice-salt bath to below 0°C, add about 40mL of 5M acetic acid solution dropwise (20 minutes to complete) (thereby adjusting the pH to 2-3), after the dropwise addition was completed, the reaction was stirred at room temperature for 2 hours. Then it was extracted with dichloromethane, the lowe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com