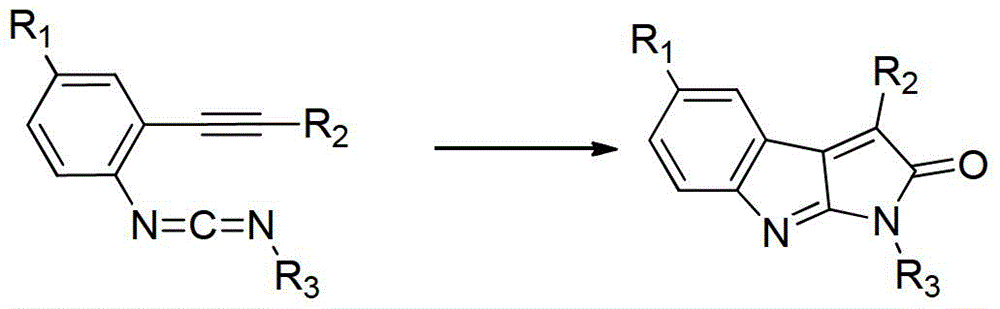
Catalytic synthetic method of pyrrolindole compounds
A synthesis method and compound technology, which is applied in the synthesis of fused ring compounds and the catalytic synthesis of pyrroloindole compounds, which can solve the problems affecting the efficiency and safety of industrial scale-up production, the use of highly toxic reagents, and the narrow scope of application of substrates, etc. problems, to achieve the effect of good application prospects and broad market promotion value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] At room temperature and under a nitrogen atmosphere, add 100 mmol of the above formula (I) compound, 140 mmol of the above formula (II) compound, 200mmol upper formula (III) compound, 15mmol composite catalyst (for 5mmolPd 2 (dba) 3 Mixture with 10mmol trimethylphosphine (hexafluoroacetylacetonate) copper), 7mmol gallium trichloride, 10mmol organic ligand 1,10-phenanthroline and 200mmol organic base 2,4-lutidine, then heated to 70°C and keep warm and fully stir for 6 hours;
[0042] After the reaction is finished, filter the reaction system, cool the filtrate to room temperature, adjust the pH value to neutral, add deionized water to fully shake and wash, then extract 2-3 times with chloroform, combine the organic phases, and dry over anhydrous magnesium sulfate. After distillation under reduced pressure, the obtained residue was separated by silica gel column chromatography, and acetone and ethyl acetate in equal volume ratio were used as washing liquids to...
Embodiment 2
[0045] Reaction formula is with embodiment 1, and concrete reaction process is as follows:
[0046] At room temperature and under a nitrogen atmosphere, add 100 mmol of the above formula (I) compound, 200 mmol of the above formula (II) compound, 300mmol formula (III) compound, 24mmol composite catalyst (for 6mmolPd 2 (dba) 3 Mixture with 18mmol trimethylphosphine (hexafluoroacetylacetonate) copper), 10mmol gallium trichloride, 20mmol organic ligand 1,10-phenanthroline and 300mmol organic base 2,4-lutidine, then heated to 85°C and keep warm and fully stir for 4 hours;
[0047] After the reaction is finished, filter the reaction system, cool the filtrate to room temperature, adjust the pH value to neutral, add deionized water to fully shake and wash, then extract 2-3 times with chloroform, combine the organic phases, and dry over anhydrous magnesium sulfate. After distillation under reduced pressure, the obtained residue was separated by silica gel column chromatography, and ...
Embodiment 3
[0050] Reaction formula is with embodiment 1, and concrete reaction process is as follows:
[0051] At room temperature and under a nitrogen atmosphere, add 100 mmol of the above formula (I) compound, 150 mmol of the above formula (II) compound, 270mmol formula (III) compound, 18mmol composite catalyst (for 5mmolPd 2 (dba) 3 Mixture with 13mmol trimethylphosphine (hexafluoroacetylacetonate) copper), 8mmol gallium trichloride, 18mmol organic ligand 1,10-phenanthroline and 300mmol organic base 2,4-lutidine, then heated to 70°C and keep warm and fully stir for 5 hours;
[0052] After the reaction is finished, filter the reaction system, cool the filtrate to room temperature, adjust the pH value to neutral, add deionized water to fully shake and wash, then extract 2-3 times with chloroform, combine the organic phases, and dry over anhydrous magnesium sulfate. After distillation under reduced pressure, the obtained residue was separated by silica gel column chromatography, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com