Preparation method of solid phosphoric acid catalyst, vibration type directional fine powder feeder applied to preparation method as well as application of feeder
A phosphoric acid catalyst, feeder technology, applied in chemical instruments and methods, physical/chemical process catalysts, feeding devices, etc., can solve the problems of difficulty in water removal, low contact probability, low reaction efficiency, etc., to improve activity and resistance. The effect of mudding performance, ensuring dispersion uniformity and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
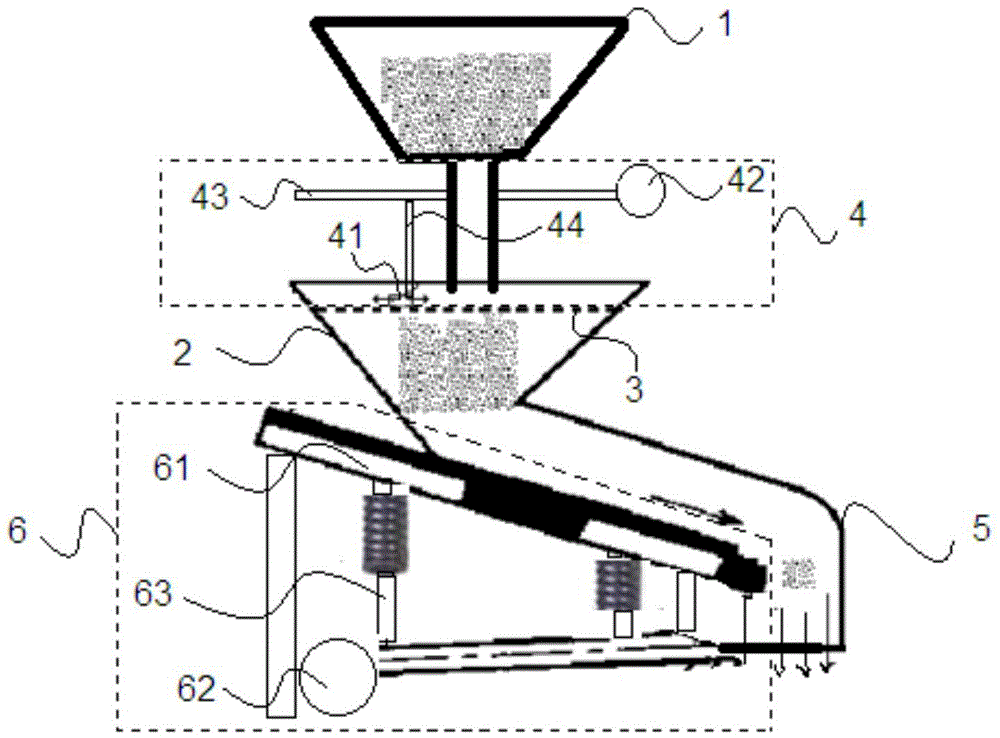
[0056] The preparation method of the solid phosphoric acid catalyst of the present invention includes: boron phosphate and borophosphoric acid formation reaction, followed by heating and kneading, homogenization, molding, drying and other processes to obtain a catalyst precursor with a diameter of 3 mm to 7 mm, followed by roasting and molding processes , activation and "pre-attrition" process to obtain the finished solid phosphoric acid catalyst.
[0057] In step (1), the above-mentioned boron phosphate and phosphoric borate generation reaction process adopts a vibratory fine powder quantitative feeder, which feeder includes a hopper, an upper feed bin, a screen (for example, an 80-mesh sieve at the bottom of the upper feed bin net) and its supporting plate, sliding brush parts on the upper part of the screen, sliding brush drive motors and guide rails, lower hoppers, and vibrating parts.
[0058] In step (1), the above-mentioned boron phosphate and boron phosphate generating...
Embodiment 1
[0059] Embodiment 1 prepares solid phosphoric acid catalyst (SPAC-1)
[0060] In the present embodiment, the preparation process of solid phosphoric acid catalyst (SPAC-1):
[0061] (1) The reaction of adding materials and generating boron phosphate: when the temperature of the reactor jacket reaches about 90°C, start stirring, add a specified amount of preheated polyphosphoric acid, continue to heat up, and reach 180°C in about 1 hour, start the oscillating fine Divide the directional feeder, adjust to a suitable feeding speed (for example, 1.0 kg / min), start adding a specified amount of boride, the reaction of generating boron phosphate is a moderately exothermic confrontation reaction, the temperature rises, and when it reaches 200 ° C, shut down The reactor jacket is electrically heated, and the temperature of the heat carrier is adjusted to keep the reaction temperature at 220°C. The water produced by the reaction must be removed from the system in time, so that the react...
Embodiment 2
[0070] Embodiment 2 prepares solid phosphoric acid catalyst (SPAC-2)
[0071] (1) The reaction of adding materials and generating boron phosphate: when the temperature of the jacket of the reactor reaches about 80°C, start stirring, add a specified amount of preheated polyphosphoric acid, continue to heat up, and reach 180°C in about 1 hour, start the oscillating fine Directional feeder, set feed rate 1.1 kg / min), start to add a specified amount of boride, the reaction of generating boron phosphate is a moderate exothermic confrontation reaction, the temperature rises, when it reaches 200 ° C, close the reactor jacket electric Heating, adjusting the temperature of the heat carrier to keep the reaction temperature at 210°C, the water produced by the reaction must be removed from the system in time, so that the reaction continues to the right, and the reaction is kept for 1 hour.
[0072](2) For the reaction of forming niobium phosphoborate, maintain a temperature of about 210°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com