New intermediate of non-small-cell lung carcinoma treating drug Ceritinib, and preparation method thereof
A technology of non-small cell lung cancer and ceritinib, which is applied in organic chemistry and other fields, and can solve the problem of low literature yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1,2-isopropylsulfide nitrobenzene
[0026] Add 1.4kg of 2-fluoronitrobenzene, 0.77kg of isopropyl mercaptan, and 1.55kg of potassium carbonate into 8kg of N,N-dimethylformamide, raise the temperature to 80-90°C for 24 hours, and monitor the completion of the reaction by HPLC. Cool down to 20-25°C, and remove insoluble matter by filtration. Add the filtrate to 76 kg of purified water at 0-5°C that is vigorously stirred, and adjust the temperature to 5-10°C to adjust the pH to 6-7. Add 15 kg of petroleum ether: ethyl acetate = 2:1 mixed solvent for extraction twice. The extract phases were combined, concentrated under reduced pressure to remove petroleum ether, until the total volume was about 10 L, and directly injected into the next step.
Embodiment 2
[0027] The preparation of embodiment 2,2-isopropylsulfide aniline
[0028] Add 400 g of Egret Z activated carbon to the ethyl acetate solution obtained in the previous step, and stir at 30 to 40° C. for 15 to 16 hours. Remove gac by filtration, wash twice with 2kg ethyl acetate. Add 140g of 10% Pd / C to the ethyl acetate solution, 30-40°C, 1.6atm catalytic hydrogenation, the reaction is completed in about 19-20 hours, and the reaction is monitored by HPLC. The catalyst was removed by filtration, concentrated to dryness under reduced pressure to obtain 1.9 kg of red oily liquid.
Embodiment 3、2
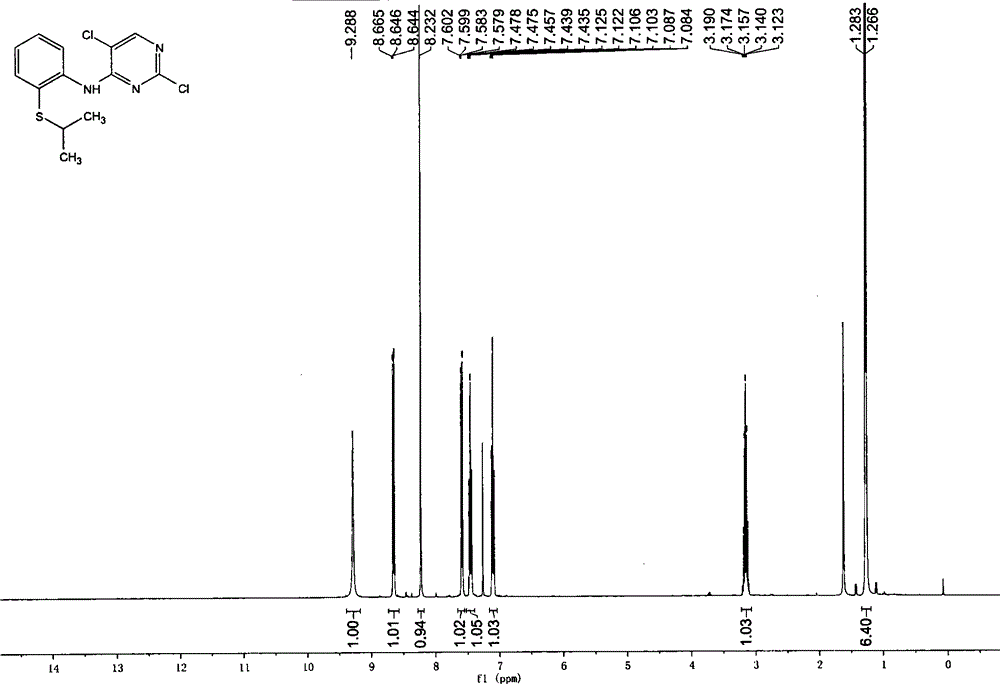
[0029] Embodiment 3, the preparation of 2,5-dichloro-N-(2-(isopropyl sulfide) phenyl) pyrimidin-4-amine
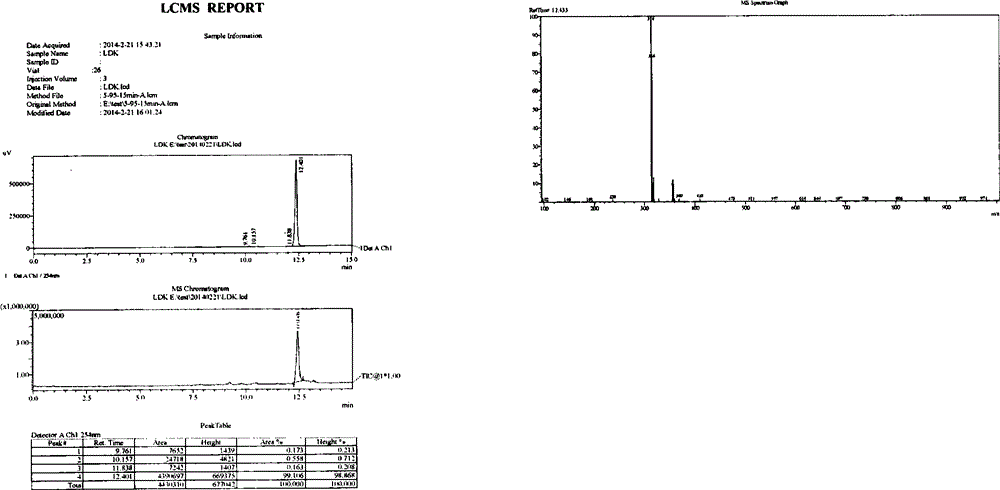
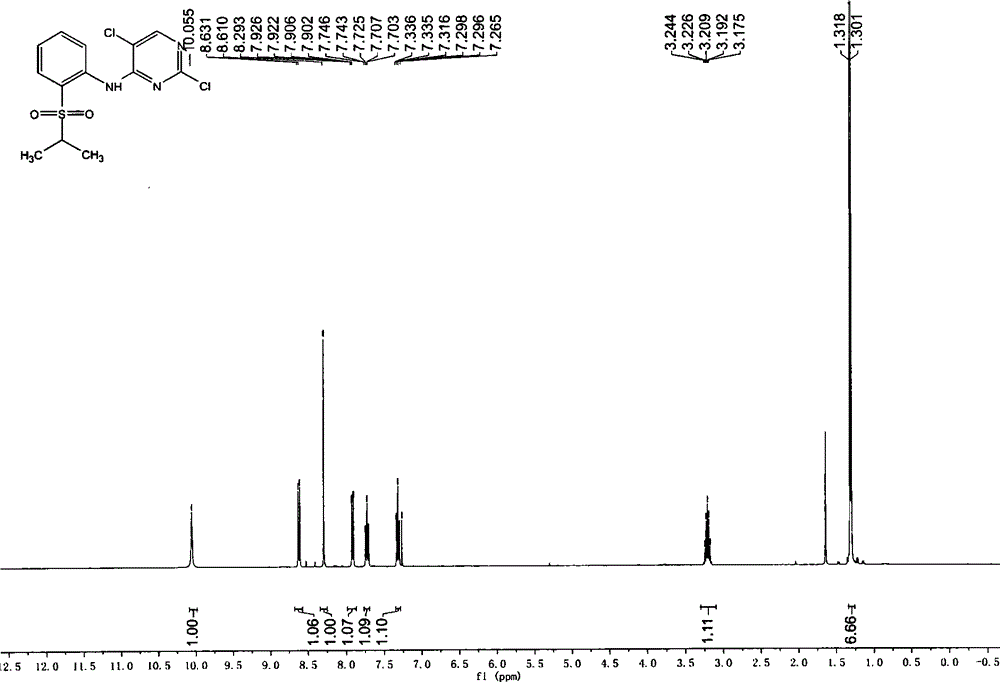
[0030] Add 1.9kg of crude product from the previous step, 1.9kg of 2,4,5-trichloropyrimidine, and 2.4kg of diisopropylethylamine into 20kg of isopropanol, and heat up to reflux for reaction. HPLC monitors the end point of the reaction until the content of the raw material 2-isopropylsulfide aniline is lower than 6%. The temperature was lowered to 40-55° C., the dry reaction solution was concentrated under reduced pressure, and 20 kg of ethyl acetate was added for entrainment once. Add 20kg ethyl acetate, wash twice with 20kg purified water. The ethyl acetate phase was concentrated to dryness under reduced pressure, 10 kg of absolute ethanol was added to the black oily matter, and the crystallization was stirred at 20-25°C for 4 hours, and at 0-5°C for 16 hours. Filter and wash once with 1 kg of absolute ethanol. Vacuum drying at 40°C yielded 2.65kg of white powder solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com