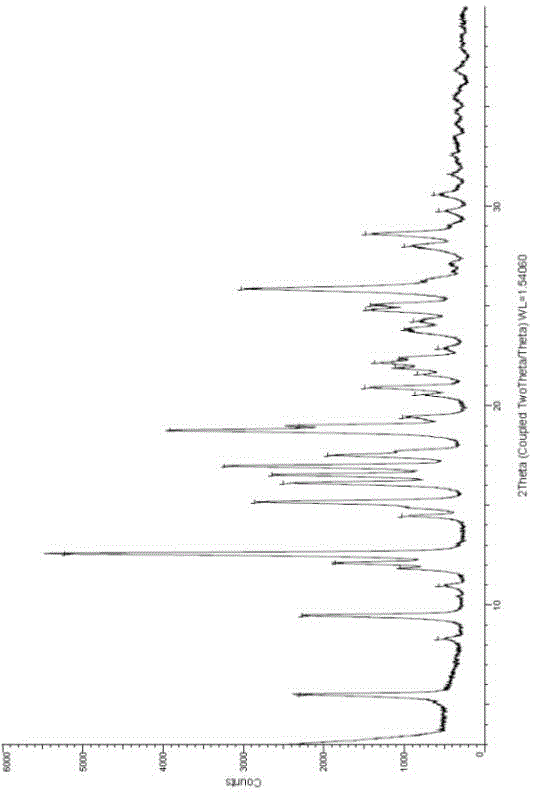
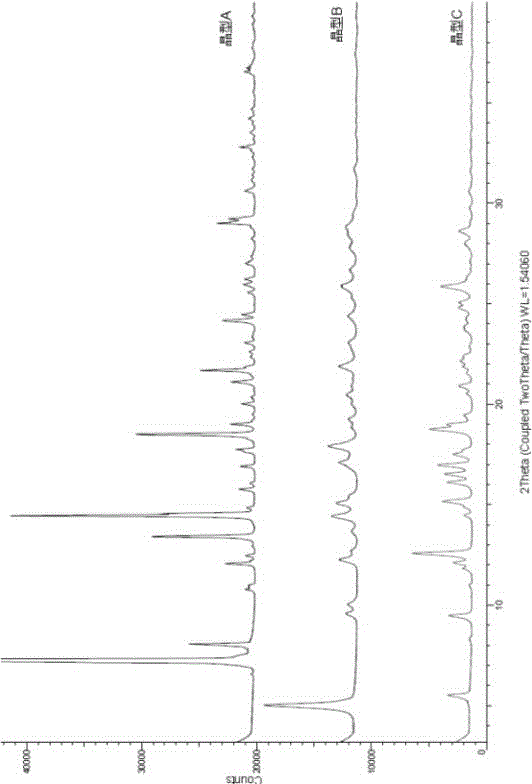
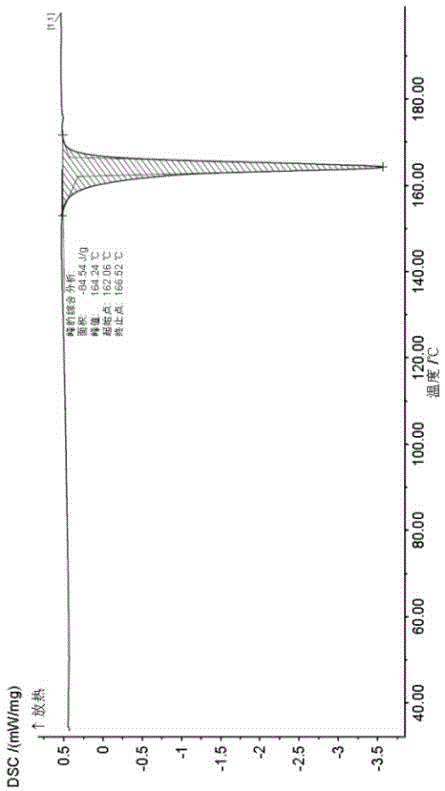
Novel crystallographic form of ceritinib and preparation method of novel crystallographic form
A technology of ceritinib and its crystal form, which is applied in the field of organic chemistry, can solve problems such as the unmentioned crystal form, and achieve the effect of stable physical and chemical properties and stable crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add ceritinib free base (100.0g) to a 2L four-neck flask (equipped with mechanical stirring, thermometer, nitrogen protection, constant pressure dropping funnel), then add purified water (1L) and stir, and control the system temperature to 20°C , then added hydrochloric acid (6N, 0.22L), and cooled down to 15°C under nitrogen protection. Sodium hydroxide solution (3N, 0.53L) was added dropwise to the reaction system, and the dropping time was controlled for 120 minutes. After a period of time, it began to slowly become turbid, and then a solid precipitated out quickly. After the solid was precipitated, the stirring was continued for 168 hours. Filter, wash the filter cake with purified water (500ml×3), vacuumize (under nitrogen protection), and place the product in a vacuum drying oven at 80°C for 96 hours to obtain ceritinib crystal C (64g, Yield 72%).
Embodiment 2
[0047] Add ceritinib (200.0g) to a 5L four-neck flask (equipped with mechanical stirring, thermometer, nitrogen protection, constant pressure dropping funnel), then add purified water (2L) and stir, control the system temperature at 10°C, add Hydrochloric acid (3N, 0.8L) was cooled to 5°C under nitrogen protection. Add potassium hydroxide solution (3N, 0.8L) dropwise to the reaction system, and the dropping time is controlled at 60 minutes. After dropping for a period of time, it slowly becomes cloudy, and then a solid precipitates out quickly. After the solid is precipitated, continue to Stir for 24 hours. Filter, wash the filter cake with purified water (1L×3), vacuumize (under nitrogen protection), and place the product in a vacuum drying oven at 100°C for 2 hours to obtain ceritinib crystal C (176g, Yield 88%).
Embodiment 3
[0049] Add ceritinib (200.0g) to a 5L four-neck flask (equipped with mechanical stirring, thermometer, nitrogen protection, constant pressure dropping funnel), then add purified water (2L) and stir, control the system temperature at 5°C, add Sulfuric acid solution (6N, 0.22L), temperature controlled to 20°C under nitrogen protection. Ammonia water (7N, 0.5L) was added dropwise to the reaction system, and the dropping time was controlled at 180 minutes. After a period of time, it began to slowly become cloudy, and then a solid precipitated out quickly, and the stirring was continued for 72 hours after the solid was precipitated. Filter, wash the filter cake with purified water (1L×3), vacuumize (under nitrogen protection), and place the product in a vacuum drying oven at 120°C for 2 hours to obtain ceritinib crystal C (170g, Yield 85%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com