Alkaloid compound and preparation method as well as application thereof
A technology of alkaloids and compounds, applied in the field of natural medicinal chemistry, can solve the problem of the lack of one in the selection and breeding of new disease-resistant varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
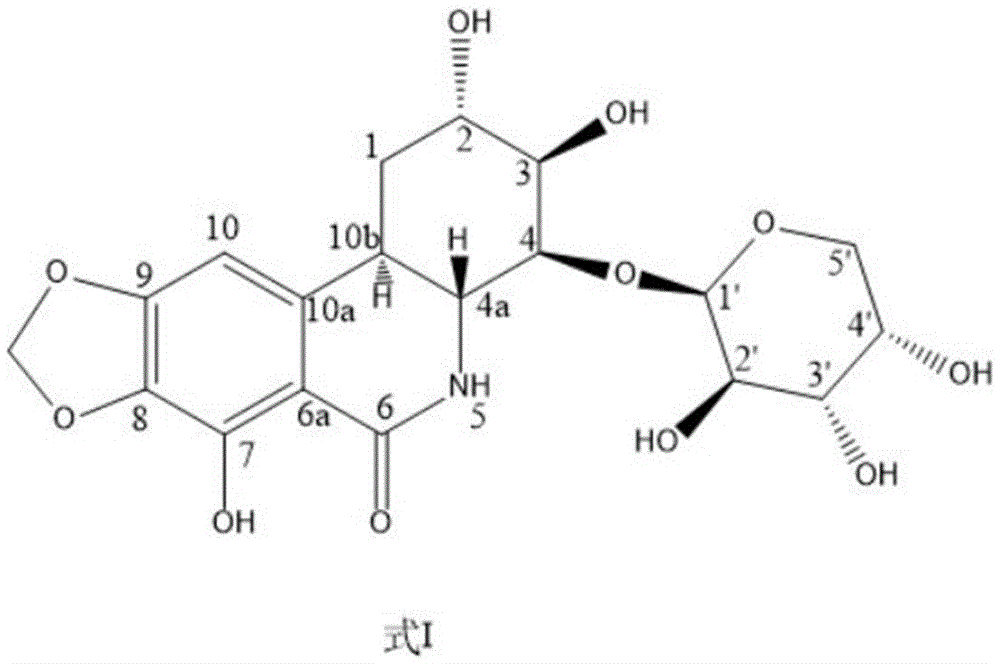
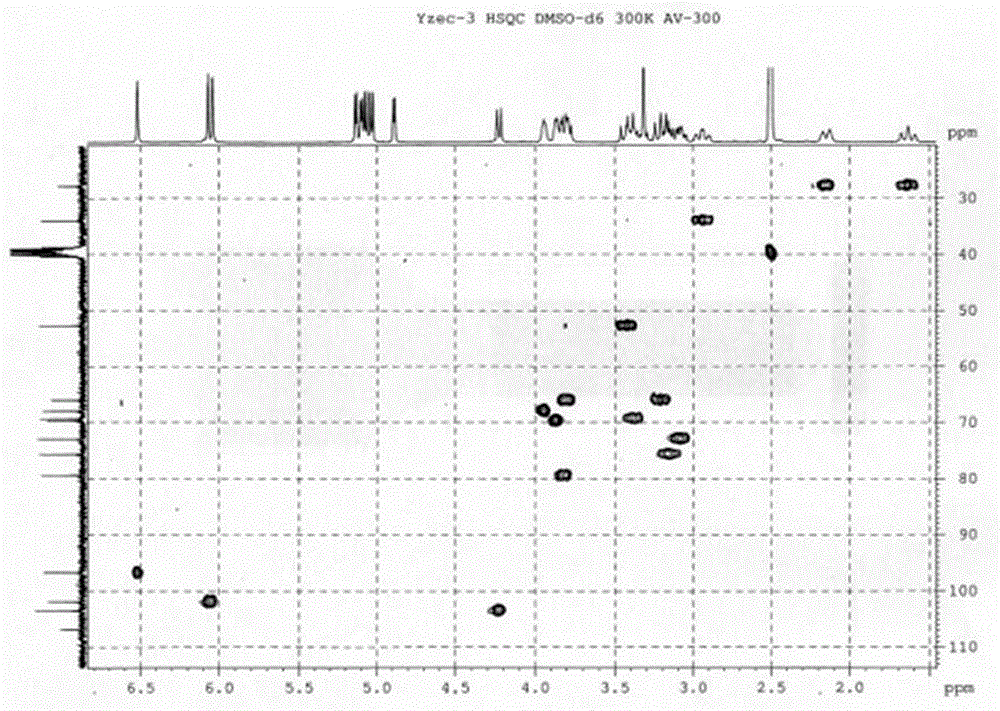
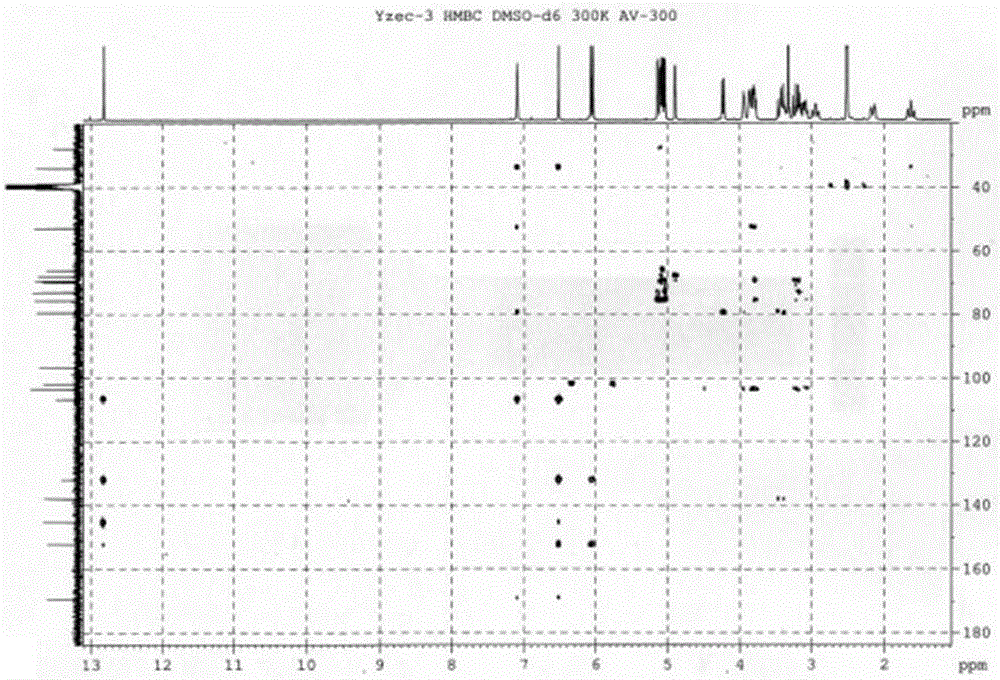
[0034]Fresh onion lotus bulbs, chopped, dried to get 40kg, extracted with 95% ethanol cold immersion 3 times, each time for 10 days, filtered to obtain the cold immersion extract, and the filter residue was extracted 4 times with 95% ethanol hot reflux, 2h / time, Combine the extracts from cold soaking and hot extracting, concentrate under reduced pressure to obtain extract, and add water to dissolve. The aqueous solution was passed through a macroporous resin D101 column and eluted with different concentrations of ethanol, and 147 g of extract was obtained from the 30% ethanol part. 147 g of the obtained extract was applied to a silica gel column, eluted with chloroform-methanol-water system for segmental and repeated column chromatography to obtain compound Yzec-3, the purity of which was detected by HPLC-MS was greater than 98%. After the structure was analyzed by mass spectrometry and nuclear magnetic resonance, it was named dihydronarciclasine-4-O-α-L-arabinoside (dihydrona...
Embodiment 2
[0042] Lycoris bulbs were extracted 3 times with 80% ethanol under heat reflux, 2h / time, filtered and combined extracts, concentrated to a small volume, dispersed with water, and subjected to macroporous resin AB-8 column chromatography, collecting 30% alcohol eluted parts, and then with reverse phase C 18 Silica gel column chromatography, eluted with 60% ethanol, repeated 3 times, obtained. The purity was greater than 90% as detected by HPLC.
Embodiment 3
[0044] Lycoris bulbs were extracted 3 times with 75% ethanol hot reflux, 2h / time, filtered and combined extracts, concentrated to a small volume, dispersed with water, and then subjected to macroporous resin D101 column chromatography to collect 30% ethanol eluted parts, and then used Silica gel column chromatography, elution with chloroform-methanol-water system, and reverse phase C 18 Silica gel column chromatography, eluted with 60% ethanol. The purity is greater than 85% as detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com